US-based cell and gene therapy (CGT) specialist Precigen, Inc. (NASDAQ: PGEN) announced that the US Food and Drug Administration (FDA) has accepted a biologics license application (BLA) for its zopapogene imadenovec (PRGN-2012) for the treatment of adults with recurrent respiratory papillomatosis (RRP). The agency is expected to act on the filing before August 27, 2025, and has also granted it priority review status.
FDA Acceptance and Priority Review
The acceptance of the BLA for PRGN-2012 marks a significant step in the regulatory process. With the priority review status, the FDA will aim to complete its review within a shorter timeframe than a standard review. This designation is given to therapies that, if approved, would provide significant improvement in the treatment, diagnosis, or prevention of a serious condition.
PRGN-2012’s Background and Designations
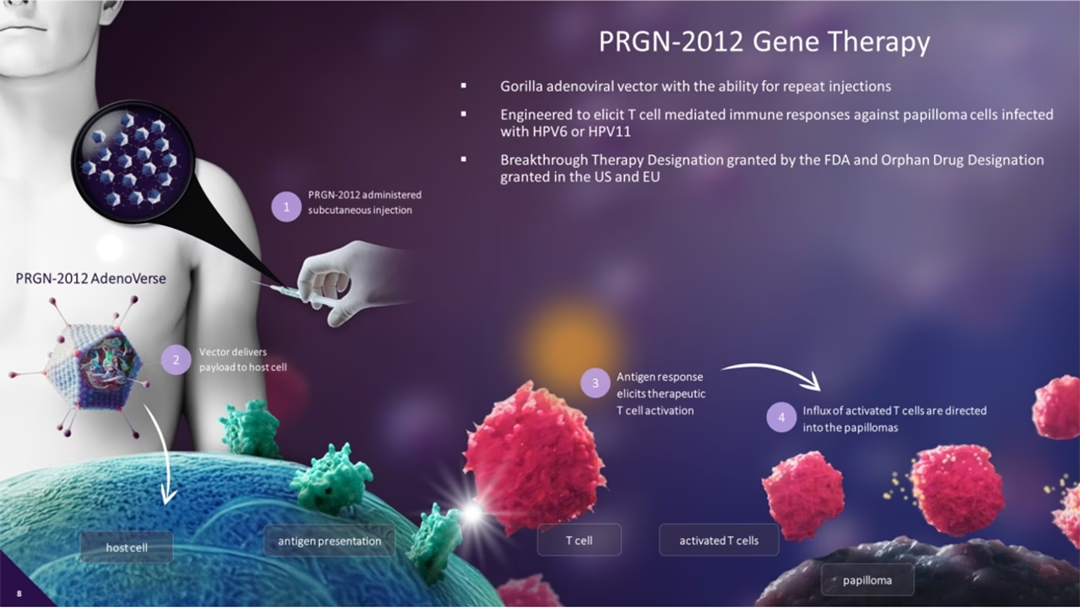
PRGN-2012 is a therapeutic human papillomavirus (HPV) 6/11 vaccine based on Precigen’s AdenoVerse technology platform. It has previously been awarded breakthrough therapy designation (BTD), orphan drug designation (ODD), and fast-track status in the US, as well as ODD in the European Union. These designations highlight the potential of PRGN-2012 to address an unmet medical need in the treatment of RRP.
Clinical Study Results
Clinical studies have demonstrated the safety and efficacy of PRGN – 2012. Over 50% of patients did not require RRP surgery within 12 months after receiving PRGN-2012 treatment, and over 85% of patients experienced a reduction in the number of surgeries compared to the year before treatment. If approved, PRGN – 2012 would be the first and only FDA-approved therapeutic for the treatment of adults with RRP.
Company Statement
“We are pleased with the FDA’s acceptance of our BLA for PRGN-2012 and the granting of priority review status,” said a spokesperson from Precigen. “This is an important milestone in our efforts to bring a potentially transformative treatment to patients suffering from RRP.”-Fineline Info & Tech