Partners Arvinas, Inc. (NASDAQ: ARVN) and Pfizer Inc. (NYSE: PFE) announced positive topline results from the Phase III VERITAC-2 study. The trial assessed vepdegestrant alone versus fulvestrant in adults with estrogen receptor-positive, human epidermal growth factor receptor 2-negative (ER+/HER2-) advanced or metastatic breast cancer who experienced disease progression following prior treatment with CDK4/6 inhibitors and endocrine therapy.
Study Results
In the study, vepdegestrant demonstrated statistically significant and clinically meaningful improvement in progression-free survival (PFS) compared to fulvestrant in the estrogen receptor 1-mutant (ESR1m) population, achieving the primary endpoint. The results exceeded the pre-specified target hazard ratio of 0.60 in the ESR1m population. Although the trial did not reach statistical significance in PFS improvement in the intent-to-treat (ITT) population, the findings represent a significant milestone for vepdegestrant.
Drug Profile and Significance
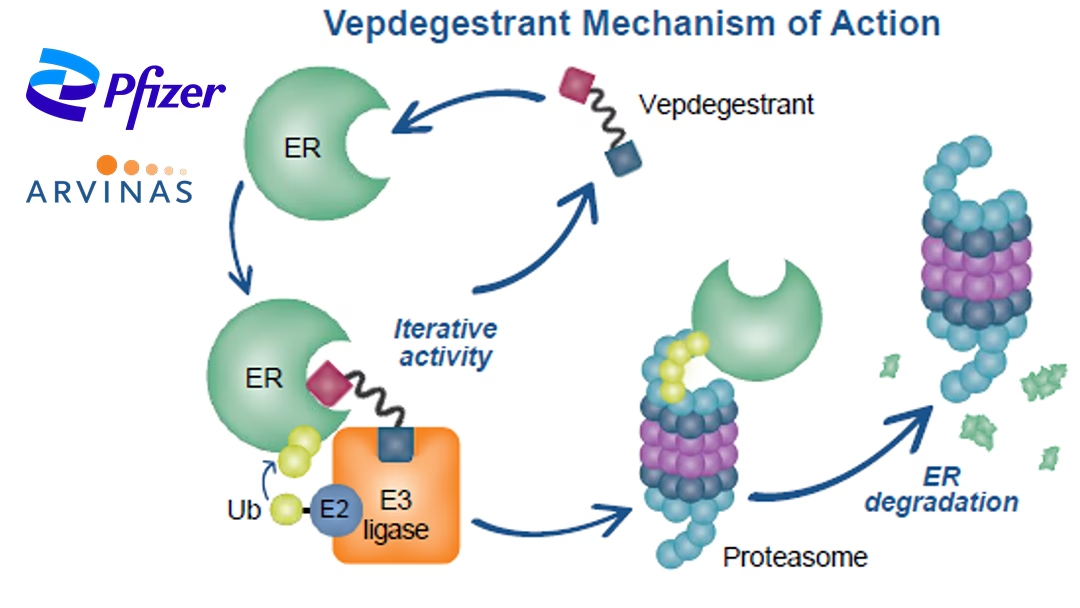
Vepdegestrant is a potential first-in-class investigational oral PROteolysis TArgeting Chimera (PROTAC) ER degrader. It was awarded fast-track status in the US in February last year for adults with ER+/HER2- advanced or metastatic breast cancer previously treated with endocrine-based therapy. These positive results mark a crucial step forward in the development of novel therapies for this patient population.-Fineline Info & Tech