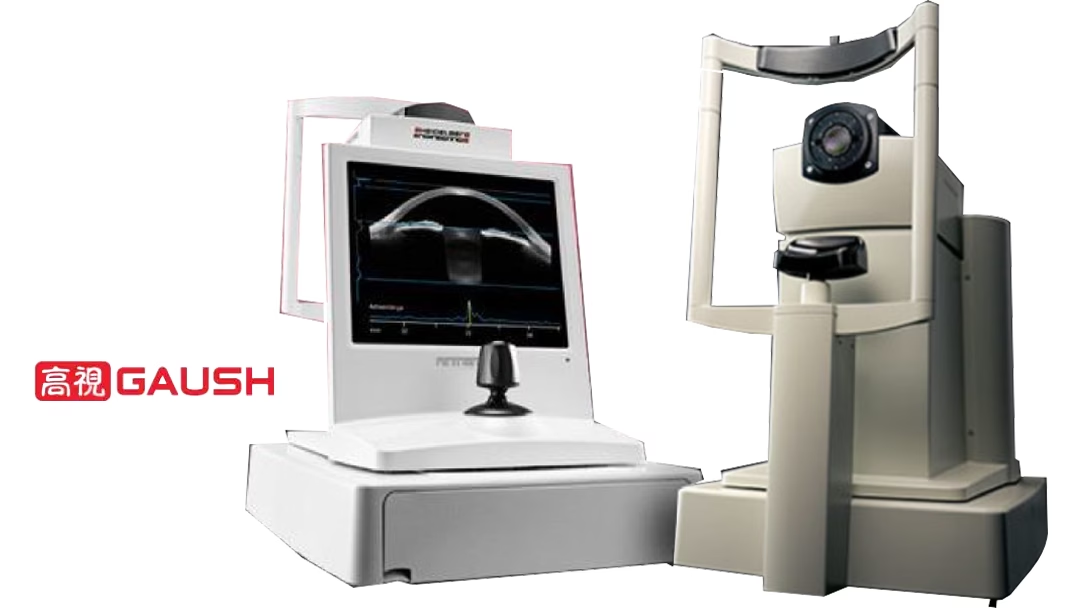
Suzhou-based Gaush Meditech Ltd (HKG: 2407) announced receiving market approval from the National Medical Products Administration (NMPA) for the ANTERION ophthalmic detector. This multimodal imaging diagnostic platform, developed by its German partner Heidelberg Instruments, integrates three functions into one device, enhancing precision and efficiency in ophthalmic diagnostics.
Technical Specifications and Applications
The ANTERION ophthalmic detector combines a biometric instrument, corneal topography map, and anterior segment optical coherence tomography (OCT) into a single platform. This integration allows for comprehensive diagnostic capabilities across multiple ophthalmic subspecialties, including refraction, corneal disease, glaucoma, and optometry.
Advanced Features
The device is distinguished by its strong penetration, fast scanning speed, and high-resolution imaging. These features, combined with eye movement tracking and ray tracing technology, ensure accurate and efficient diagnosis of anterior segment conditions. The comprehensive data provided by the ANTERION platform supports clinicians in making informed treatment decisions.-Fineline Info & Tech