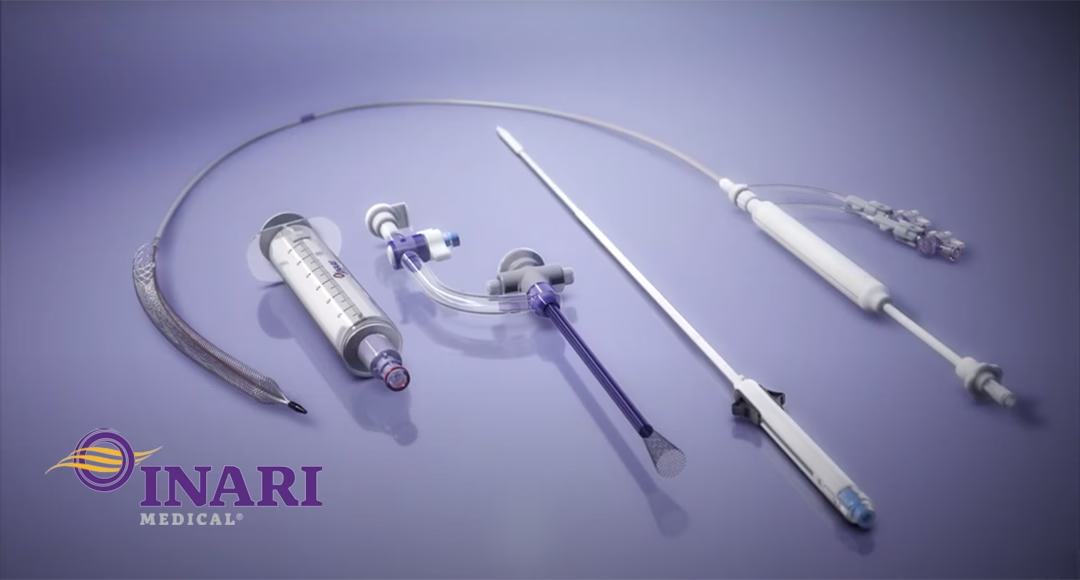
US-based Inari Medical, Inc. (NASDAQ: NARI), set to be acquired by Stryker (NYSE: SYK), announced last week that it has obtained marketing approval from China’s National Medical Products Administration (NMPA) for its peripheral vein thrombectomy stent system. This marks a significant milestone in the company’s global expansion strategy.
Product Details
The approved product consists of the ClotTriever thrombectomy sheath and ClotTriever thrombectomy stent. It is designed for transcatheter thrombectomy treatment of lower extremity deep vein thrombosis (DVT) in patients with acute iliofemoral and femoral popliteal vein DVT, as well as subacute iliofemoral vein DVT.
Partnership with VFLO Medical
In December last year, China-based VFLO Medical entered into a partnership with Inari Medical, securing distribution rights for Inari’s series of products in Greater China, including the ClotTriever system. This collaboration aims to enhance the accessibility and availability of advanced thrombectomy solutions for patients in the region.-Fineline Info & Tech