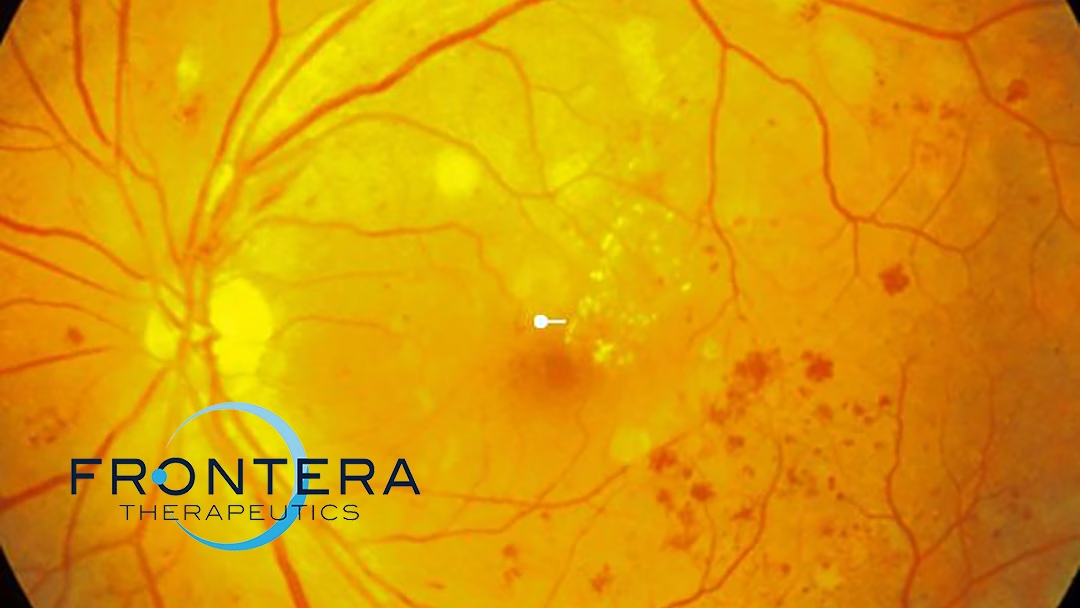
Sino-US firm Frontera Therapeutics, Inc. announced receiving approval from China’s National Medical Products Administration (NMPA) to initiate a clinical study for its FT-003, a recombinant adeno-associated virus (rAAV) gene therapy for diabetic retinopathy (DR). This follows previous approvals for studies in neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME) in China.
Background on Diabetic Retinopathy
Diabetic retinopathy (DR) is a leading cause of vision loss and blindness globally, affecting 30-35% of diabetes patients. Current treatments, primarily anti-VEGF drugs, offer intervention at various stages of DR to maintain retinal structure and prevent neovascularization.
FT-003 Mechanism and Preclinical Data
FT-003 features a clear mechanism of action. Preclinical data shows that after injection into the fundus, FT-003 efficiently transfects multiple layers of retinal cells. This prompts the expression and secretion of humanized recombinant fusion proteins similar to aflibercept, inhibiting endothelial cell division, reducing vascular permeability, and providing comprehensive therapeutic effects. The drug is designed for a single injection with long-term effectiveness.-Fineline Info & Tech