China-based Triastek Inc., a pharmaceutical-focused 3D printing company, has announced receiving clinical approval in the US for its T20G. This 3D-printed non-vitamin K antagonist oral anticoagulant (NOAC) was previously cleared for trials in China in January of last year.
T20G’s Technological Advantages
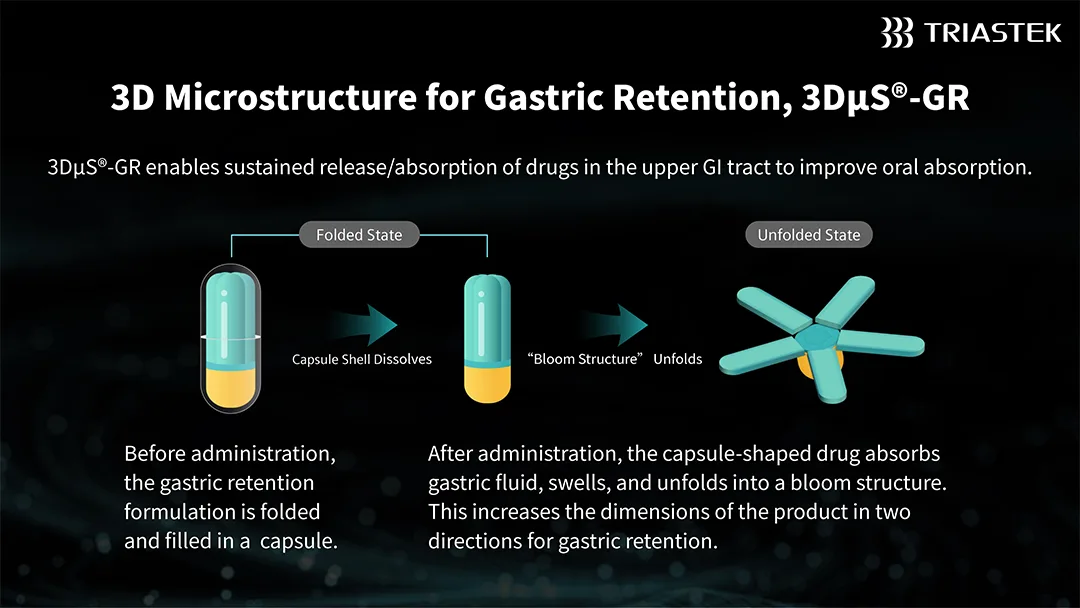
T20G leverages Triastek’s patented 3D Microstructure for Gastric Retention (3DμS®-GR) platform, combined with the company’s proprietary Melt Extrusion Deposition with Micro-Injection Molding (MED&MIM) process. This innovative approach enables once-daily oral administration, outperforming the reference listed drug (RLD) that requires twice-daily dosing. The optimized regimen is expected to enhance patient adherence and simplify dosing management.
Enhanced Drug Absorption and Efficacy
Furthermore, T20G facilitates sustained release of the active pharmaceutical ingredient (API) during its gastric retention phase. This mechanism allows for improved drug absorption in the upper gastrointestinal tract, significantly boosting its oral absorption efficiency and overall therapeutic efficacy.-Fineline Info & Tech