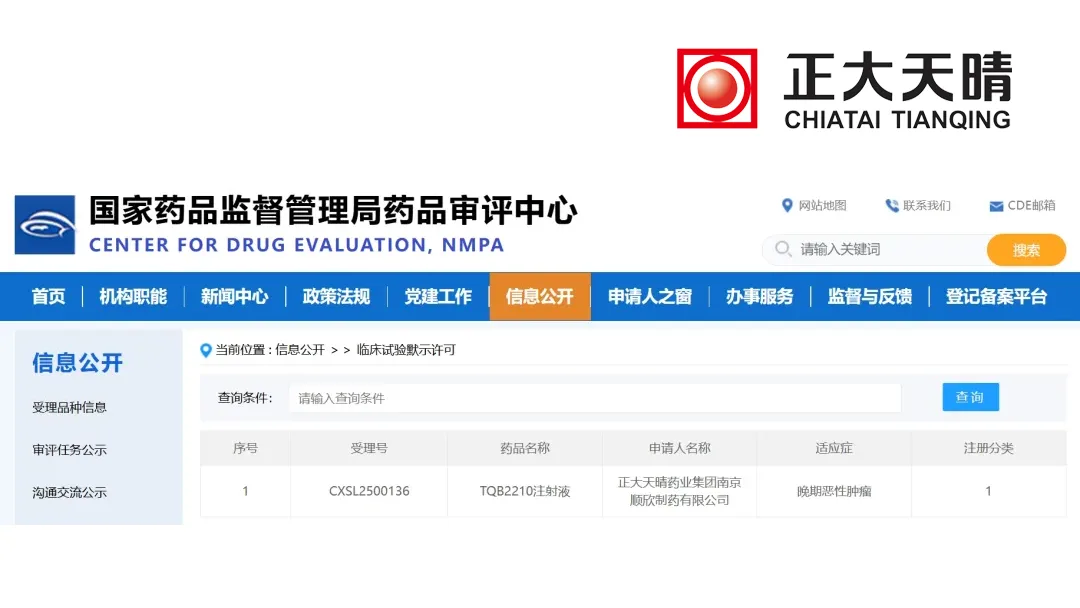
The Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) has approved Chia Tai Tianqing’s Category 1 drug TQB2210 for clinical study in advanced malignant tumors. This marks a significant step forward for the company in the field of oncology.
Drug Profile and Innovation
TQB2210 is a human monoclonal antibody (mAb) targeting FGFR2b, with no similar product approved globally. Preclinical studies have demonstrated that TQB2210 exhibits a better anti-tumor effect than or comparable to bemarituzumab. When combined with penpulimab, the drug showed improved efficacy in subcutaneous transplantation tumor models of human gastric cancer in nude mice.-Fineline Info & Tech