Sanofi (EPA: SAN, NASDAQ: SNY) announced that a market filing for its tolebrutinib as a treatment for non-relapsing secondary progressive multiple sclerosis (nrSPMS) has been accepted for review by the US FDA. The agency is expected to make its decision by September 28, 2025. A regulatory submission is also under review in the European Union (EU).
Drug Mechanism and Innovation
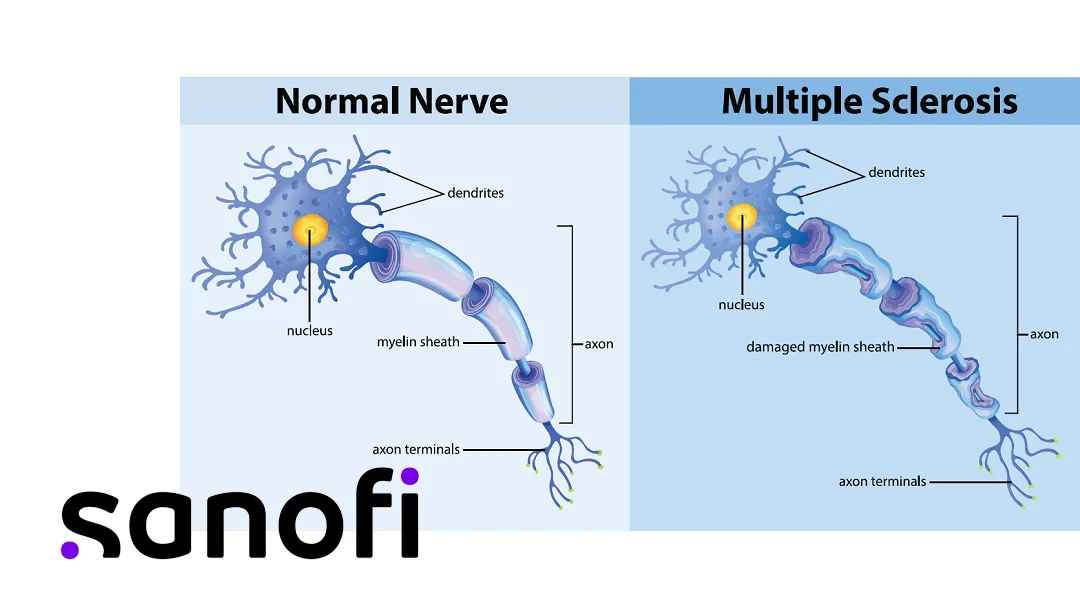
Tolebrutinib is an investigational, oral, brain-penetrant, and bioactive Bruton’s tyrosine kinase (BTK) inhibitor specifically designed to target smoldering neuroinflammation, a key driver of disability progression in multiple sclerosis (MS). Unlike currently approved MS therapies that primarily address peripheral inflammation, tolebrutinib uniquely crosses the blood-brain barrier to achieve therapeutic cerebrospinal fluid concentrations. This allows it to modulate B-lymphocytes and disease-associated microglia within the central nervous system (CNS).
Clinical Trial Progress
The drug is currently undergoing clinical trials in various forms of MS, highlighting its potential as a novel therapeutic option for patients with different disease presentations.-Fineline Info & Tech