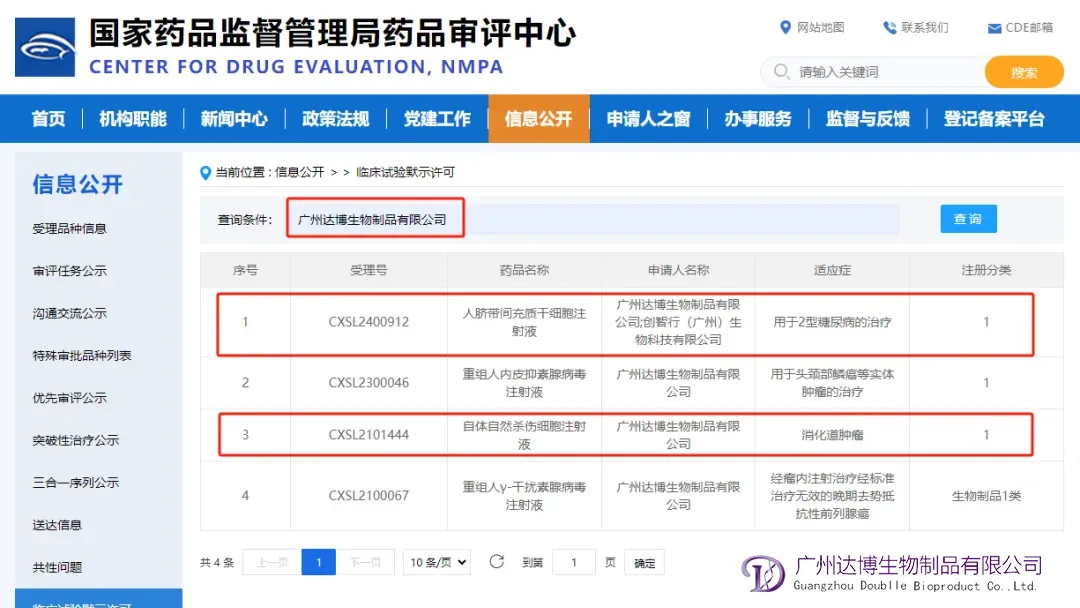
Guangzhou Doublle Bioproduct Co., Ltd. has announced receiving Investigational New Drug (IND) clearance from China’s National Medical Products Administration (NMPA) for its E101, a human umbilical cord mesenchymal stem cells (HUC-MSCs) product. The approval allows the company to initiate clinical trials for the treatment of type 2 diabetes (T2D).
Product Innovation
E101 is an in-house developed cryopreserved stem cell preparation made from umbilical cord tissue through in vitro isolation, screening, and large-scale three-dimensional amplification processes. This product marks a significant advancement as the first umbilical cord mesenchymal stem cells product in China produced using three-dimensional culture, fully automated, and large-scale processes.-Fineline Info & Tech