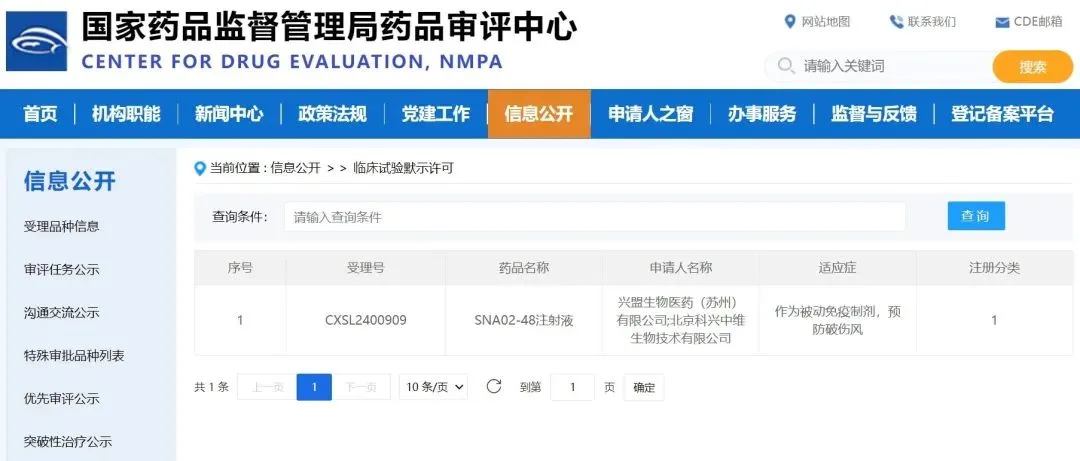
Beijing Sinovac Biotech Co., Ltd’s (NASDAQ: SVA) subsidiary Sinovac Life Sciences Co., Ltd. and its partner Synermore Biologics announced that their Category I new drug, SNA02-48 injection, has received clinical approval from the National Medical Products Administration (NMPA). This recombinant fully human monoclonal antibody targets tetanus toxin and offers a promising alternative to traditional serum products for post-tetanus exposure prophylaxis.
Disease Background
Non-neonatal tetanus is a severe, acute disease caused by toxins from Clostridium tetani. It is characterized by sustained muscle contractions and spasms. The disease has a high mortality rate, especially without medical intervention, and poses significant health risks globally.
Current Treatment Limitations
Current passive immunization options include tetanus human immunoglobulin (HTIG) and equine-derived tetanus antitoxin. These products, derived from human or animal plasma, face supply shortages and potential pathogen contamination risks.
Innovative Solution
SNA02-48, developed using Sinovac’s proprietary human antibody library platform, addresses these limitations. As a fully human monoclonal antibody, it reduces the risk of allergic reactions and serum sickness, making it particularly suitable for individuals sensitive to animal-derived products.
Clinical Progress
The approval marks a significant step toward offering a safer and more effective treatment option for tetanus prophylaxis. SNA02-48 is set to undergo further clinical evaluation to confirm its efficacy and safety.-Fineline Info & Tech