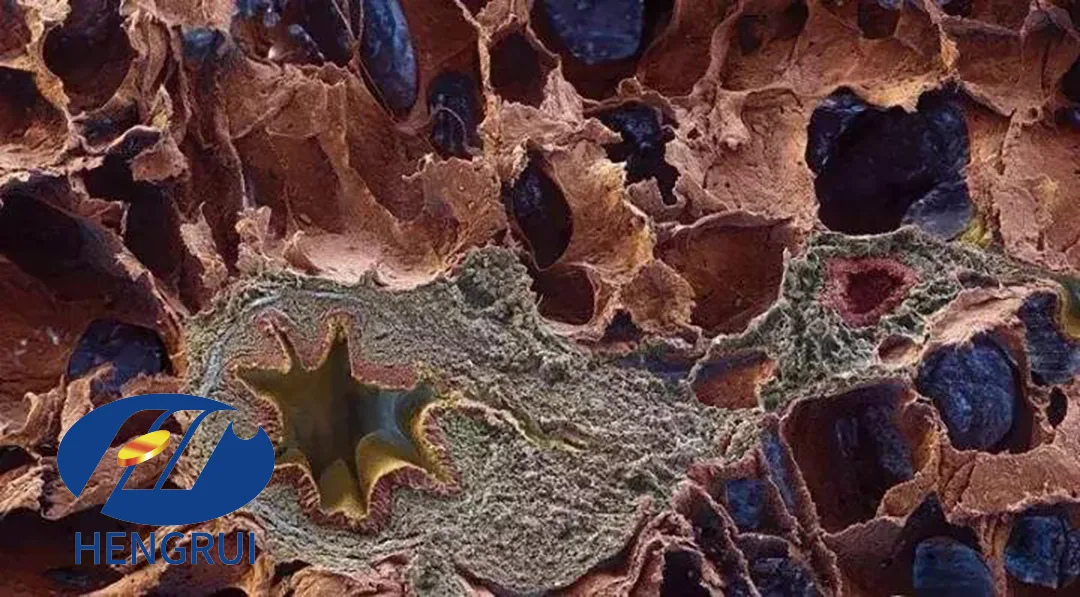
Hengrui Pharmaceuticals (SHA: 600276) announced on April 1, 2025, that its HRS-9813 capsule has received clinical trial approval from the National Medical Products Administration (NMPA). The drug is intended for the treatment of idiopathic pulmonary fibrosis (IPF), a condition with significant unmet medical needs.
Innovative Drug Profile
HRS-9813 is a Class 1 innovative drug independently developed by Hengrui Pharmaceuticals. Preclinical studies demonstrated that HRS-9813 significantly improves lung function and reduces pulmonary fibrosis in mice, with a favorable safety profile.
Global Market Potential
Currently, there are no similar drugs approved for marketing globally, positioning HRS-9813 as a potential first-in-class therapy for IPF. This approval marks a significant step forward in Hengrui’s commitment to developing novel treatments for respiratory diseases.-Fineline Info & Tech