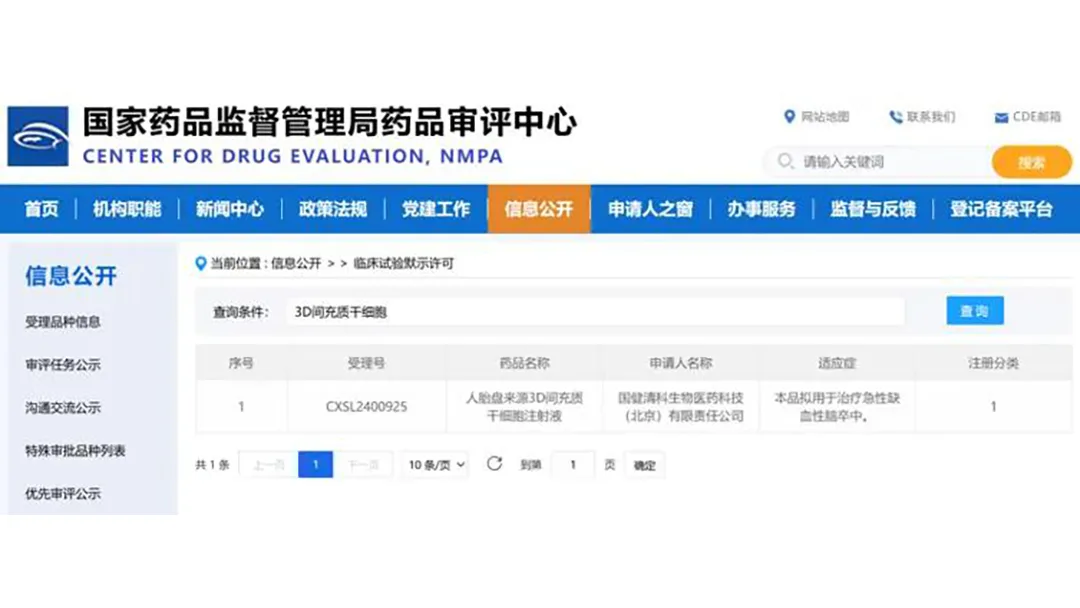
China-based Guojian Qingke Biomedical Technology (Beijing) Co., Ltd. announced that it has received tacit clinical approval from the National Medical Products Administration (NMPA) for its human placenta-derived 3D mesenchymal stem cells (MSCs) injection. This approval paves the way for clinical trials evaluating the drug’s efficacy in treating acute ischemic stroke.
Product Innovation
The stem cell product, developed using patented technology from Tsinghua University, employs a carrier-free suspension culture medium method based on fermentation tanks. This advanced preparation technique results in 3D MSCs that are smaller in size, exhibit high migration ability, and possess robust immune regulatory and tissue repair capabilities compared to traditionally prepared MSCs.
Clinical Trial Outlook
The intravenously administered therapy is designed to leverage the unique advantages of 3D MSCs to address the significant unmet medical needs in acute ischemic stroke treatment. The company is preparing to initiate clinical trials to further evaluate the safety and efficacy of this innovative approach.-Fineline Info & Tech