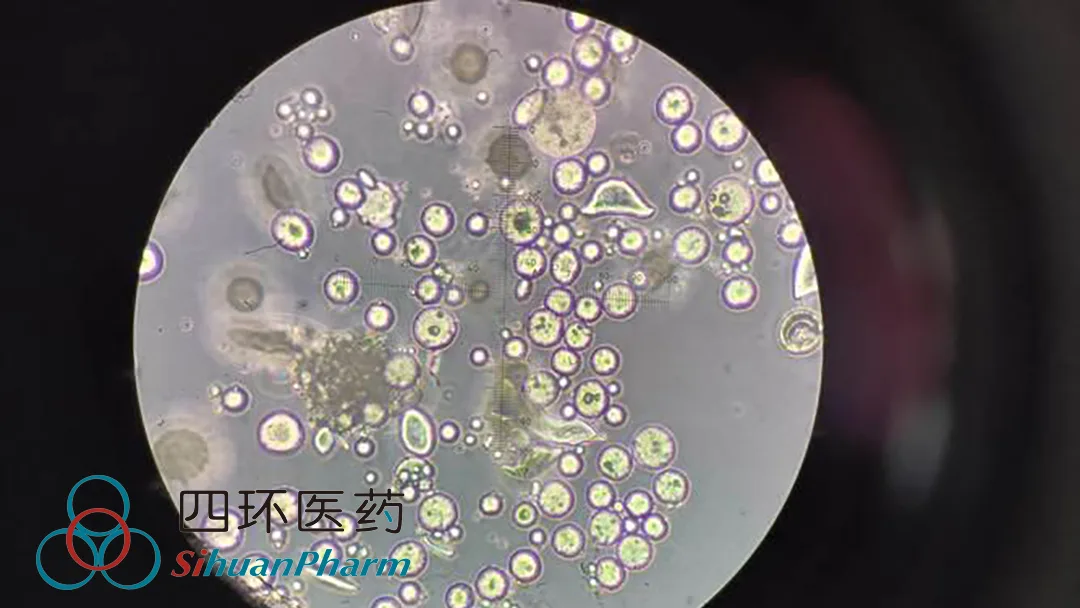
China’s Sihuan Pharmaceutical Holdings Group Ltd (HKG: 0460) announced that it has received Category III medical device licensing from the National Medical Products Administration (NMPA) for its polycaprolactone microsphere facial filler, an injectable developed by its subsidiary MEIYOUNG. This approval marks a significant milestone in the company’s expansion into the aesthetic medical sector.
Product Innovation
The polycaprolactone microsphere facial filler is a regenerative injection material designed to address facial depression. It features a unique dual-action mechanism: the gel carrier provides immediate volumetric correction upon injection, while the microspheres stimulate collagen production over time, achieving a sustained filling effect. This innovative approach combines instant aesthetic improvement with long-term benefits, setting it apart in the competitive facial aesthetics market.
Market Potential
With the growing demand for non-surgical cosmetic procedures, Sihuan’s new filler is poised to capture a significant share in the aesthetic medical device market. The product’s approval underscores the company’s commitment to advancing regenerative aesthetics and providing healthcare professionals with effective tools for facial contouring.-Fineline Info & Tech