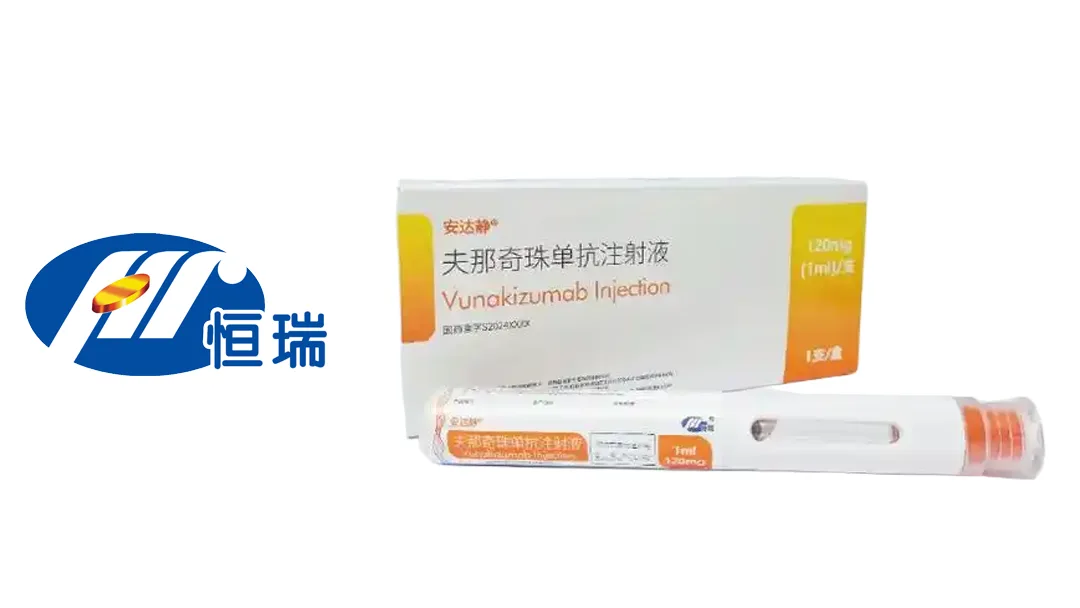
Jiangsu Hengrui Pharmaceuticals Co., Ltd. (SHA: 600276) announced that it has received additional indication approval from China’s National Medical Products Administration (NMPA) for vunakizumab, an IL-17A monoclonal antibody. The drug is now approved to treat active ankylosing spondylitis in patients who have shown a poor response to conventional treatment.
Drug Mechanism
Vunakizumab targets the IL-17 pathway, which plays a key role in several autoimmune diseases. By blocking IL-17A, the drug helps reduce inflammation and associated symptoms.
Market Expansion
This approval follows the drug’s previous approval in August 2024 for moderate to severe plaque psoriasis. The expanded indication underscores Hengrui’s commitment to addressing unmet medical needs in autoimmune conditions.-Fineline Info & Tech