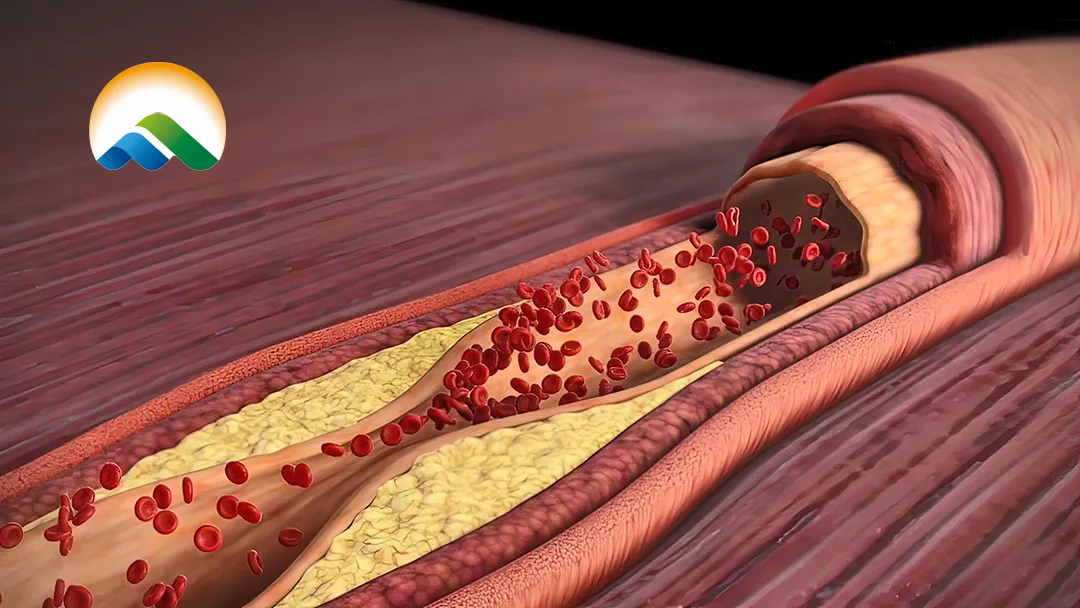
China-based Beijing Winsunny Pharmaceutical Co., Ltd (SHA: 601089) announced that it has received clinical approval from the National Medical Products Administration (NMPA) for its Category 1 chemical drug FY101, administered via subcutaneous injection. This marks a significant milestone in the development of innovative therapies for hyperlipidemia.
Drug Profile
FY101 is a first-in-class chemical drug designed to address hyperlipidemia, a condition characterized by elevated levels of lipids in the blood. The drug’s novel mechanism of action and subcutaneous administration route offer potential advantages in patient compliance and therapeutic outcomes.
Global Innovation
With no similar products approved globally, FY101 represents a breakthrough in the treatment landscape for hyperlipidemia. This approval underscores Winsunny’s commitment to developing innovative solutions for cardiovascular health.-Fineline Info & Tech