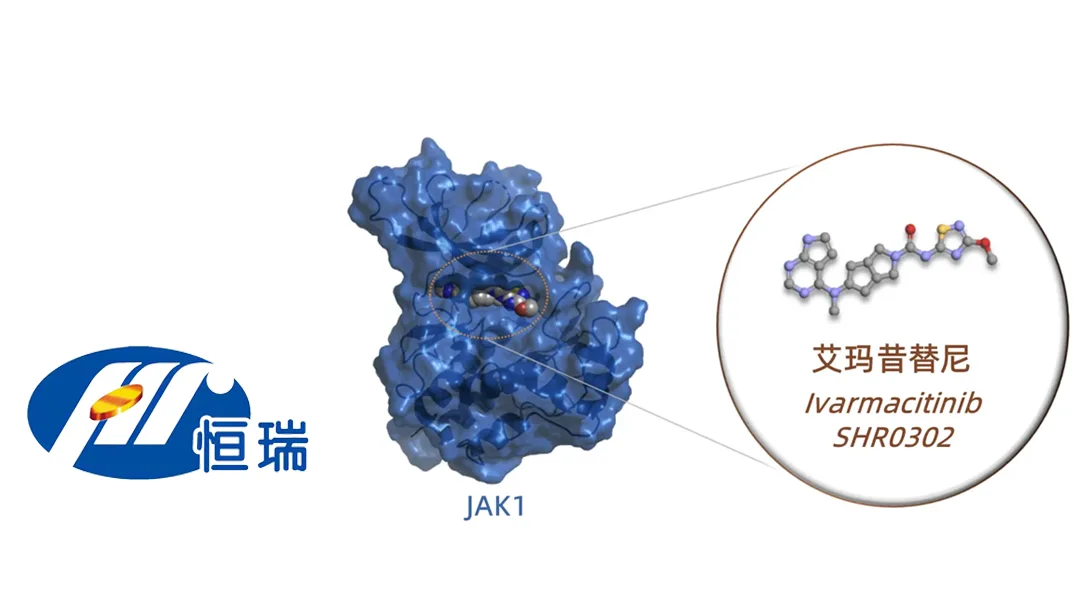
Jiangsu Hengrui Pharmaceuticals Co., Ltd. (SHA: 600276) announced that it has received additional indication approval from China’s National Medical Products Administration (NMPA) for its Category 1 drug ivarmacitinib. The approval allows the JAK1 inhibitor to treat adult patients with moderate to severe atopic dermatitis (AD) who have not responded adequately or are intolerant to existing topical or systemic therapies.
Drug Mechanism
Ivarmacitinib is a highly selective JAK1 inhibitor, which works by blocking the Janus kinase 1 (JAK1) pathway, a key driver in inflammatory and immune-mediated diseases. This mechanism of action makes it effective in reducing inflammation and managing symptoms associated with atopic dermatitis.
Previous Approvals
Earlier this year, ivarmacitinib received NMPA approval for the treatment of active ankylosing spondylitis (AS) in March, followed by approval for moderate to severe active rheumatoid arthritis (RA) in April. These approvals highlight the drug’s versatility in addressing various autoimmune conditions.-Fineline Info & Tech