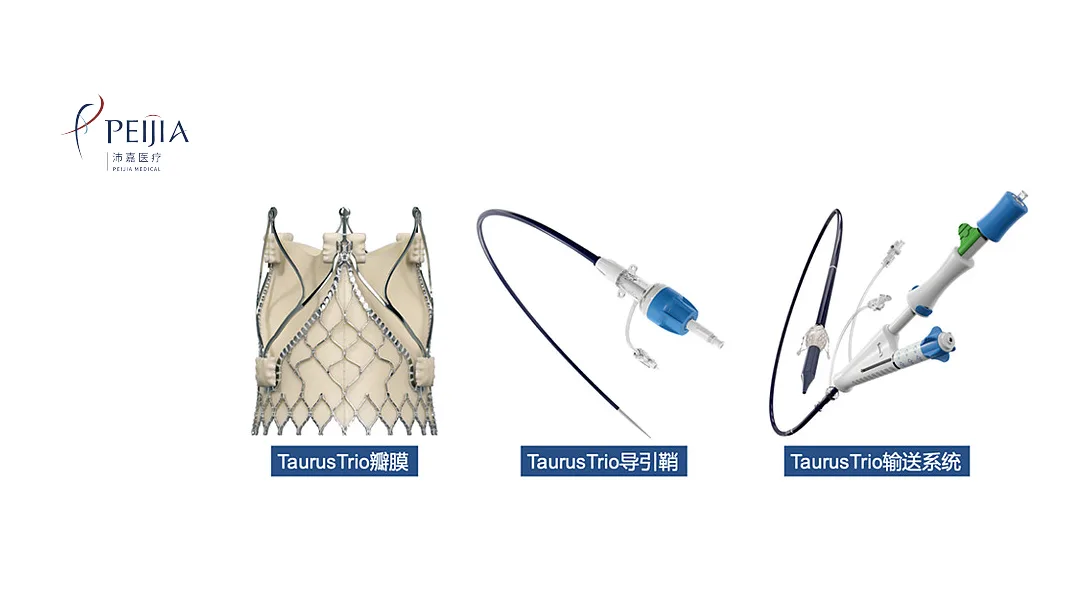
China-based Peijia Medical Ltd (HKG: 9996) has announced that the National Medical Products Administration (NMPA) has accepted for review a market filing for its TaurusTrio transcatheter aortic valve (TAV) system. This marks a significant step toward potential market approval for the innovative device designed to treat severe aortic valve regurgitation.
TaurusTrio System: Innovation and Application
The TaurusTrio system is part of the Trilogy Heart Valve System licensed from US-based JenaValve Technology, Inc. It is specifically designed for patients with primary severe aortic valve regurgitation (AR) and can be delivered via femoral artery access. This minimally invasive approach offers a new treatment option for patients who may not be candidates for traditional open-heart surgery.
Regulatory Milestone
The TaurusTrio system was awarded fast-track status by the NMPA in June 2023, highlighting its potential to address an unmet medical need in China. The acceptance of the market filing for review brings Peijia Medical closer to commercializing this advanced technology and expanding its presence in the structural heart disease market.-Fineline Info & Tech