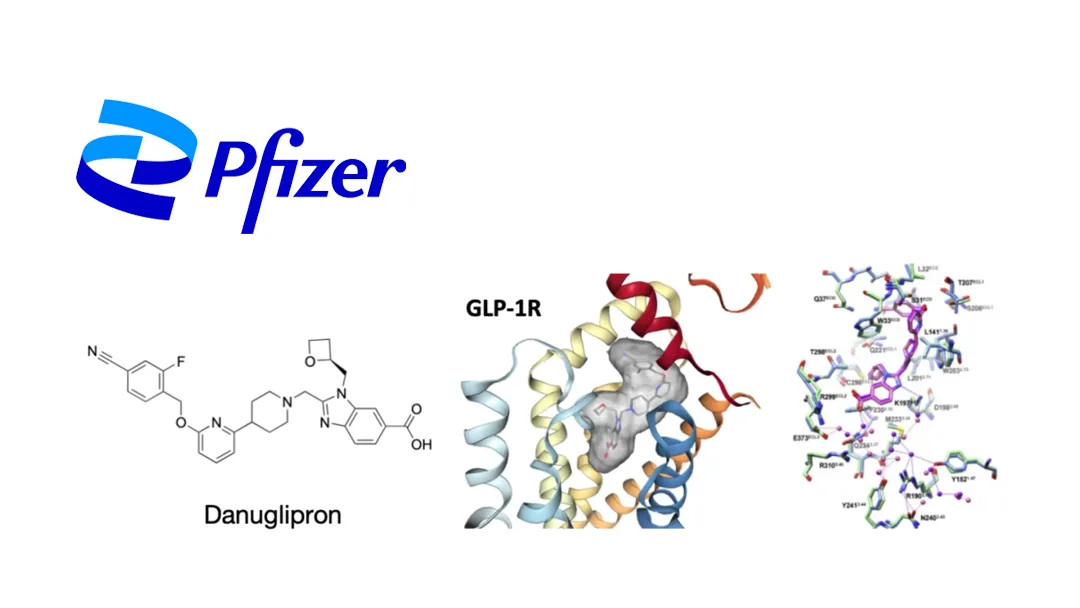
US pharmaceutical giant Pfizer Inc. (NYSE: PFE) has announced the termination of development for its oral GLP-1 receptor agonist danuglipron (PF-06882961) following a case of drug-induced liver injury (DILI) observed in a clinical trial participant. The hepatic abnormality resolved after discontinuation of the treatment, but Pfizer concluded that the risk-benefit profile no longer supported further development.
Danuglipron: Development and Clinical Profile
Danuglipron was developed as an oral glucagon-like peptide-1 (GLP-1) receptor agonist for the treatment of type 2 diabetes (T2D) and obesity. Pfizer prioritized a once-daily formulation to enhance competitiveness, building on earlier twice-daily versions that demonstrated efficacy. Two dose-optimization studies (NCT06567327, NCT06568731) met pharmacokinetic objectives and identified a potentially competitive Phase III dose.
Clinical Trial Findings and Decision
Across over 1,400 participants, liver enzyme elevations occurred at rates comparable to those seen with approved GLP-1 therapies. However, a single asymptomatic case of DILI, which resolved post-discontinuation, prompted Pfizer to reassess the drug’s risk-benefit profile. After reviewing all clinical data and regulatory feedback, Pfizer decided to halt further development of danuglipron.
Market Impact and Future Outlook
The termination of danuglipron highlights the challenges in advancing GLP-1 therapies, particularly in balancing efficacy with safety. Pfizer’s decision underscores the rigorous evaluation processes required to ensure patient safety and regulatory compliance. The company will now focus its resources on other promising candidates in its pipeline.-Fineline Info & Tech