China-based Jiangsu Hengrui Pharmaceuticals Co., Ltd. (SHA: 600276) has announced clearance from the National Medical Products Administration (NMPA) to conduct a Phase II study evaluating the safety, tolerability, and efficacy of HRS-7058 in combination with adebrelimab (SHR-1316), bevacizumab, and SHR-1826 for the treatment of advanced solid tumors. This marks a significant step in the development of innovative therapies targeting KRAS G12C mutations.
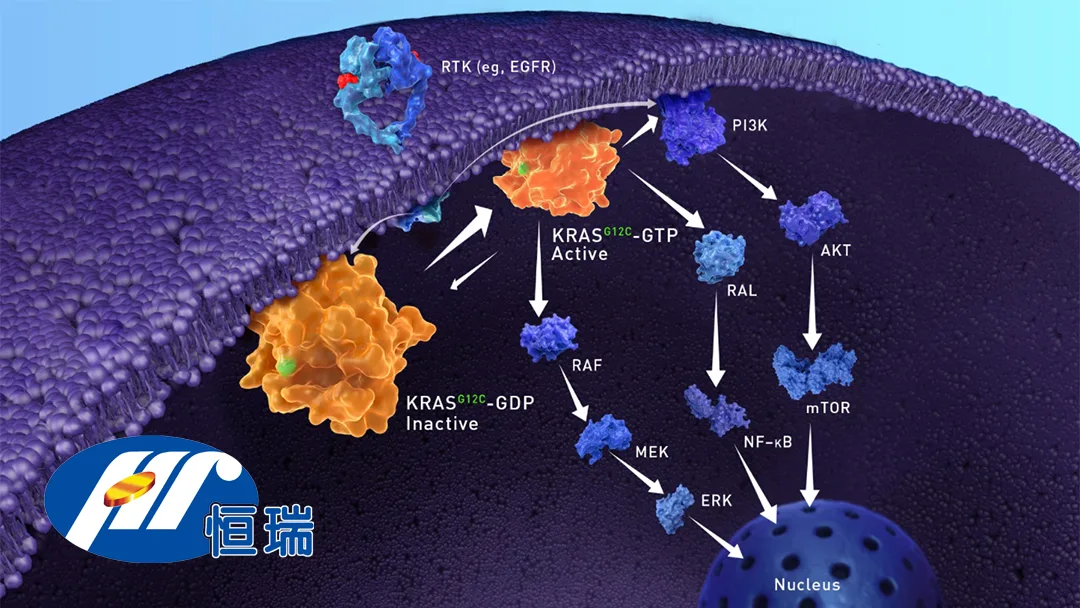
HRS-7058: Targeting KRAS G12C Mutations
HRS-7058 is a novel selective inhibitor of the KRAS G12C mutation, designed to treat advanced solid tumors. Similar products include Innovent Biologics’ Dupert (fulzerasib) and Sino Biopharmaceutical’s garsorasib (D-1553), both approved in China, as well as Amgen’s sotorasib, which is commercially available internationally. HRS-7058 aims to offer a competitive alternative in this rapidly evolving therapeutic space.
Combination Therapy and Mechanism
The Phase II study will assess HRS-7058 in combination with adebrelimab (a PD-L1 monoclonal antibody), bevacizumab (an anti-VEGF antibody), and SHR-1826 (a c-MET-targeted antibody-drug conjugate). This multi-targeted approach leverages complementary mechanisms to enhance anti-tumor efficacy. SHR-1826, in particular, stands out as a first-in-class ADC with no approved equivalents globally, further underscoring the innovation in Hengrui’s pipeline.
Market Context and Future Outlook
With several KRAS G12C inhibitors already approved or in late-stage development, Hengrui’s entry into this competitive landscape highlights its commitment to addressing unmet medical needs. The combination therapy’s potential to improve patient outcomes in advanced solid tumors positions Hengrui favorably in both domestic and international markets.-Fineline Info & Tech