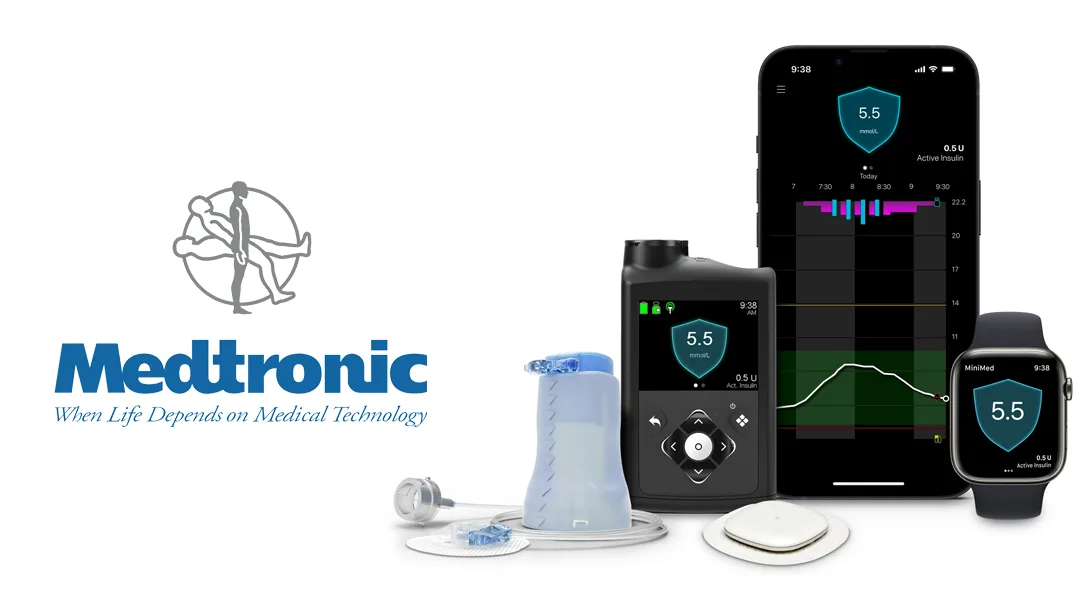
US-Irish medical technology firm Medtronic plc (NYSE: MDT) has secured marketing approval from the US Food and Drug Administration (FDA) for its Simplera Sync sensor, designed for use with the MiniMed 780G system. This approval introduces greater flexibility for users of Medtronic’s advanced insulin delivery system, which features Meal Detection technology compatible with both the Guardian 4 sensor and the newly approved Simplera Sync sensor.
Simplera Sync: Features and Benefits
The Simplera Sync is a disposable, all-in-one sensor that eliminates the need for fingersticks when used with SmartGuard or overtape. It boasts a straightforward, two-step insertion process, enhancing user convenience. As the latest addition to Medtronic’s continuous glucose monitoring (CGM) portfolio, the Simplera Sync broadens options for users, offering enhanced flexibility and ease of use.
Market and Clinical Impact
The approval of the Simplera Sync sensor underscores Medtronic’s commitment to advancing diabetes care through innovative technology. By providing multiple sensor options, the MiniMed 780G system can better meet the diverse needs of patients managing insulin-dependent diabetes.-Fineline Info & Tech