China-based Sichuan Biokin Pharmaceutical Co., Ltd (SHA: 688506) has received approval from the National Medical Products Administration (NMPA) to conduct two Phase II studies for its innovative bispecific antibody-drug conjugate (ADC) BL-B01D1. The trials will evaluate the drug in combination therapies for extensive-stage small cell lung cancer (ES-SCLC) and advanced malignant tumors of the biliary tract.
Clinical Trial Details
The first study will investigate BL-B01D1 in combination with a PD-1/PD-L1 inhibitor, with or without anlotinib and chemotherapy, for ES-SCLC. The second study will assess the drug in combination with durvalumab, with or without chemotherapy, for advanced biliary tract tumors. These trials highlight the potential of BL-B01D1 to enhance treatment outcomes through strategic combination therapies.
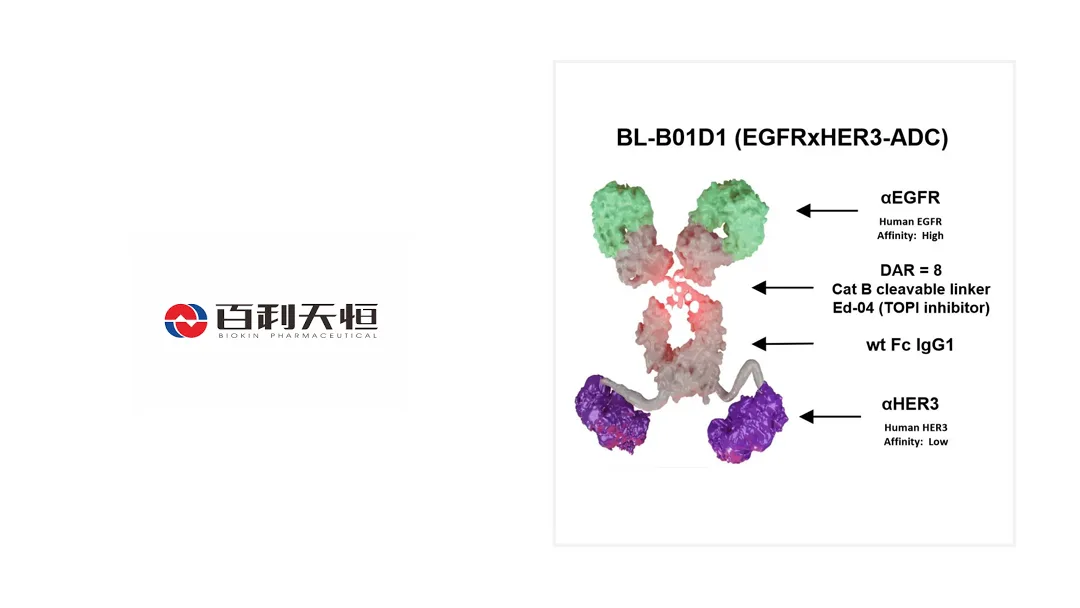
BL-B01D1: Global Leadership in EGFR/HER3 Targeting
BL-B01D1 is the world’s only EGFR/HER3 bispecific ADC at the Phase III stage. It is being evaluated in nearly 40 studies across more than 10 tumor types in China and the United States, underscoring its broad therapeutic potential and Biokin’s commitment to innovative oncology solutions.-Fineline Info & Tech