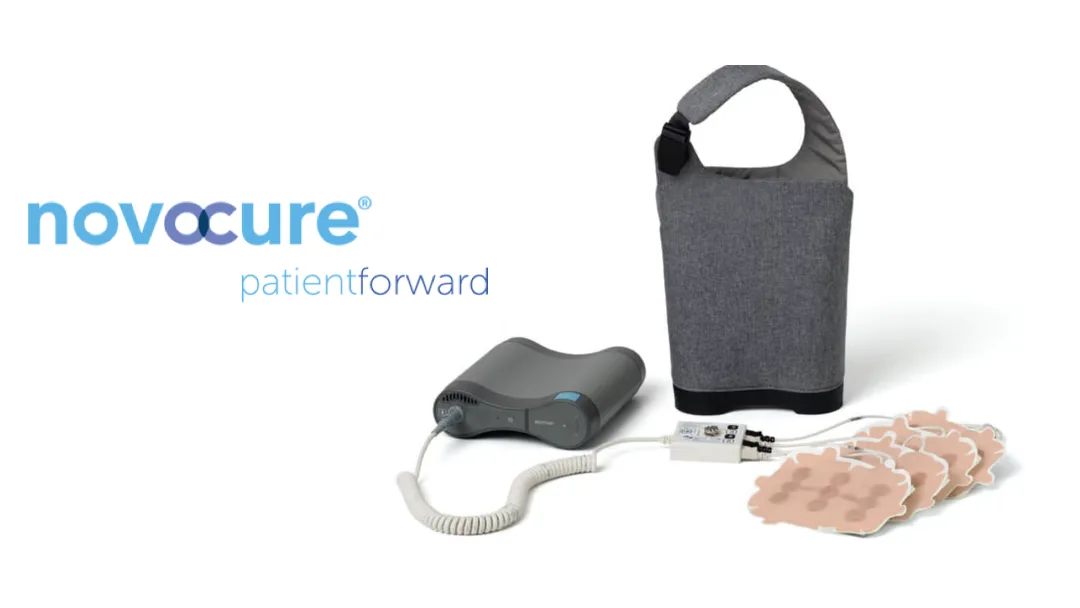
Switzerland-based Novocure (NASDAQ: NVCR), the developer of Tumor Treating Fields (TTFields) for cancer therapy, has announced CE mark approval for its Optune Lua device. This wearable technology is now approved for use in adult patients with metastatic non-small cell lung cancer (NSCLC) who have progressed after platinum-based treatment and are receiving immune checkpoint inhibitors or docetaxel.
Optune Lua: Mechanism and Innovation
Optune Lua delivers TTFields through non-invasive, wearable arrays. These alternating electric fields disrupt cancer cell division, leading to cell death while minimizing impact on healthy cells. The device received FDA approval in the US in October 2024 and has now secured CE mark recognition in the EU.
Clinical Evidence and Impact
The CE mark approval is supported by the Phase III LUNAR study, which demonstrated improved median overall survival (OS) of 13.2 months for patients treated with Optune Lua in combination with immune checkpoint inhibitors or docetaxel, compared to 9.9 months for those receiving only the latter therapies. This advancement offers a new therapeutic option for patients with metastatic NSCLC.-Fineline Info & Tech