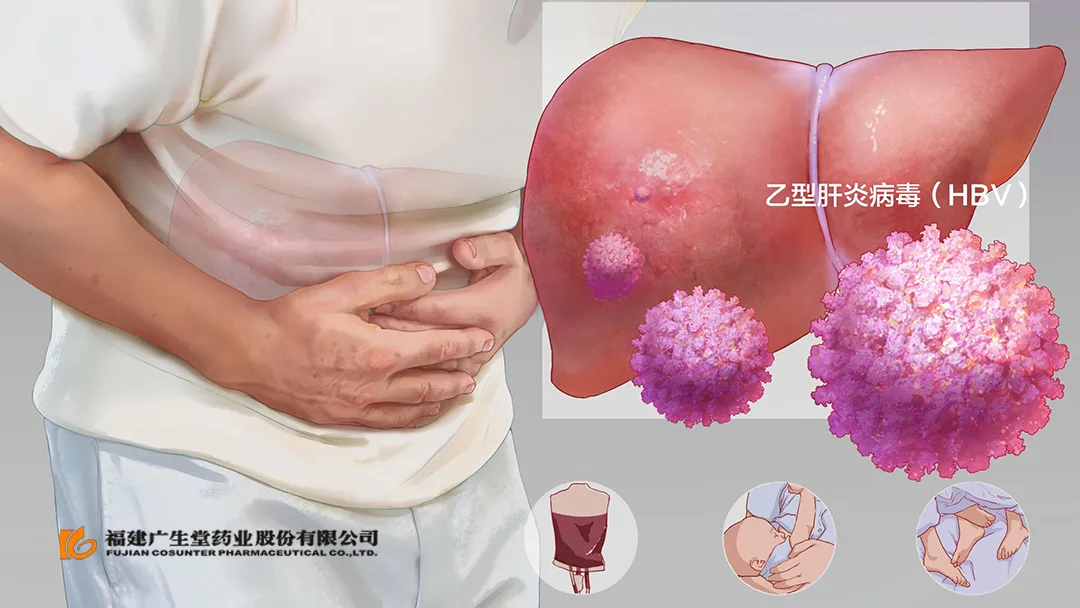
China-based Fujian Cosunter Pharmaceutical Co., Ltd (SHE: 300436) announced that it has received clearance from the National Medical Products Administration (NMPA) to conduct a Phase II study evaluating the safety, tolerability, and efficacy of its GST-HG131 combined with neracorvir (GST-HG141) in patients with chronic hepatitis B virus (HBV). Both products are Category 1 drugs, indicating their innovative nature.
Mechanism and Efficacy
Previous preclinical and clinical research have demonstrated that GST-HG131 significantly inhibits hepatitis B surface antigen (HBsAg), while GST-HG141 effectively reduces hepatitis B virus deoxyribonucleic acid (HBV DNA) and pregenomic RNA (pgRNA). This reduction indirectly reflects the potential for effective inhibition and depletion of hepatitis B virus covalently closed circular DNA (HBV cccDNA). The combination of these two drugs is expected to enhance antiviral efficacy through complementary mechanisms.-Fineline Info & Tech