Zhejiang-based Zhejiang Shimai Pharmaceutical Co.,Ltd (CentryMed)’s CMDE005, an anti-EGFR×CD3 enzyme-controlled bispecific antibody (BsAb) developed using the company’s proprietary proBiTE platform, has obtained tacit clinical approval from the US Food and Drug Administration (FDA). This approval paves the way for clinical evaluation of CMDE005 in multiple EGFR-positive advanced solid tumors.
CMDE005 Mechanism and Advantages
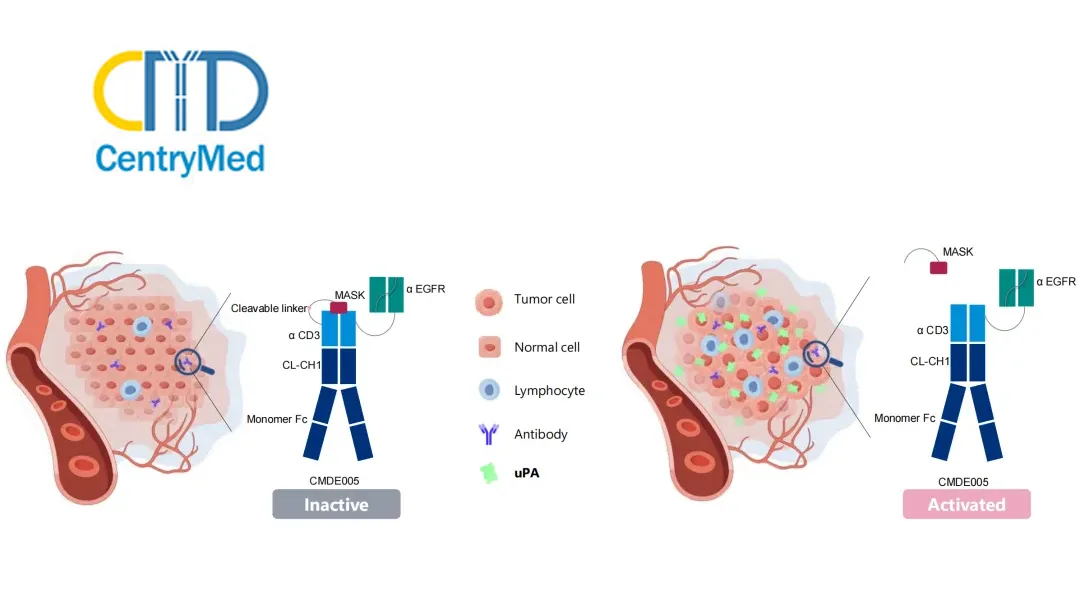
CMDE005 is designed with a unique safety profile. In peripheral blood and normal tissues, the anti-CD3 end of CMDE005 remains in an inactive state. This innovative design minimizes the risk of non-specific activation and associated toxicities. Upon entering tumor tissue, tumor-specific expression proteases such as urokinase-type plasminogen activator (uPA) cleave the CMDE005-specific site. This cleavage results in the dissociation of the “shielding peptide,” releasing its binding function to CD3. Consequently, CMDE005 is converted into an active EGFR×CD3 bispecific antibody, enabling targeted anti-tumor activity while avoiding toxic side effects from non-specific killing of normal tissue. This mechanism underscores CMDE005’s high tumor-targeting capability and potential for improved safety and efficacy in treating EGFR-positive advanced solid tumors.-Fineline Info & Tech