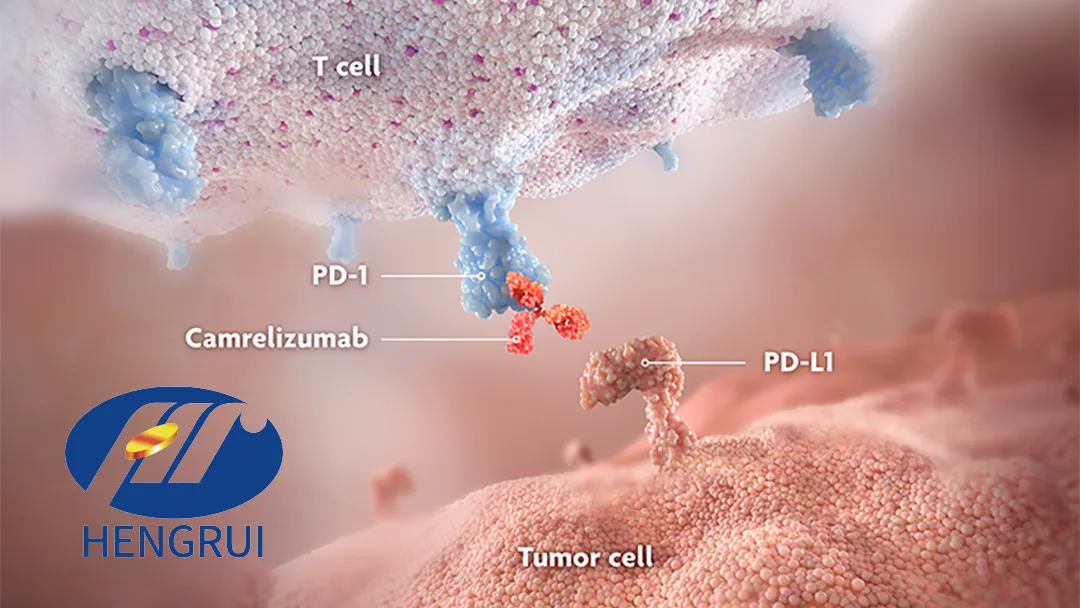
China-based Jiangsu Hengrui Pharmaceuticals Co., Ltd. (SHA: 600276) announced that it has received clinical clearance from the National Medical Products Administration (NMPA) for its programmed death-1 (PD-1) monoclonal antibody (mAb) camrelizumab. The drug will now be assessed in combination with apatinib plus temozolomide for the treatment of first-line advanced acral melanoma (AM). The study protocol also includes a comparison of camrelizumab with dacarbazine in first-line advanced AM.
Clinical Trial Significance
This marks the tenth clinical indication for camrelizumab, further solidifying Hengrui’s leading position in PD-1/L1 targeted therapies. Camrelizumab now has nine approved indications in China, including six for non-small cell lung cancer (NSCLC), three for gastrointestinal cancers, and it is the first immune checkpoint inhibitor targeting acral melanoma in China.-Fineline Info & Tech