Sino-US biotech company Frontera Therapeutics, Inc. announced that its recombinant adeno-associated virus (rAAV) gene therapy FT-017, targeting hypertrophic cardiomyopathy (HCM) caused by MYBPC3 mutations, has received clinical trial approvals from both China’s National Medical Products Administration (NMPA) and the U.S. Food and Drug Administration (FDA).
Understanding Hypertrophic Cardiomyopathy (HCM)
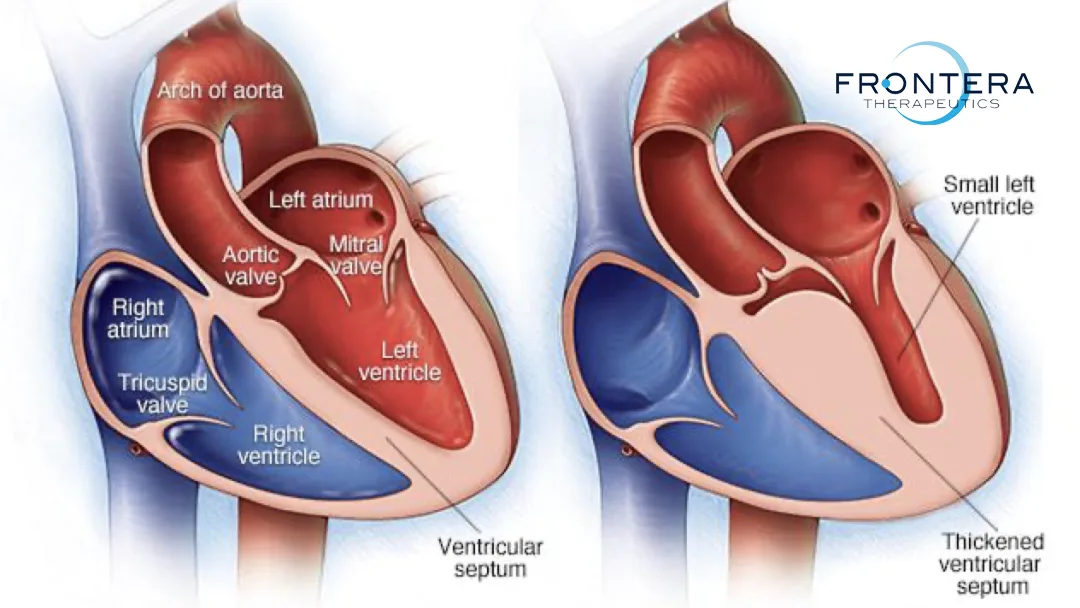
Hypertrophic cardiomyopathy (HCM) is a primary cardiomyopathy characterized by ventricular wall thickening, primarily left ventricular hypertrophy. As the most common inherited heart disease, HCM is strongly associated with complications such as atrial fibrillation, stroke, heart failure, and sudden cardiac death. The primary cause lies in pathogenic mutations of genes encoding sarcomeric proteins, such as the cardiac myosin-binding protein C gene (MYBPC3). These mutations lead to deficient synthesis of functional proteins, resulting in structural and functional abnormalities of sarcomeres, histopathological changes (e.g., cardiomyocyte hypertrophy, disorganized fiber arrangement, interstitial fibrosis), and progressive myocardial remodeling. Current therapies, which focus on symptom management and myosin contraction inhibition, only provide temporary relief and do not address the genetic defects or prevent disease progression or associated risks of arrhythmias and sudden death.
FT-017: Targeting the Root Cause of HCM
FT-017 addresses the root cause of MYBPC3-associated HCM by delivering a codon-optimized human MYBPC3 gene via intravenous infusion. The therapy enables long-term, stable expression of functional cardiac myosin-binding protein C (cMyBP-C) in cardiomyocytes, aiming to restore normal sarcomere structure and function and reverse pathological myocardial remodeling.-Fineline Info & Tech