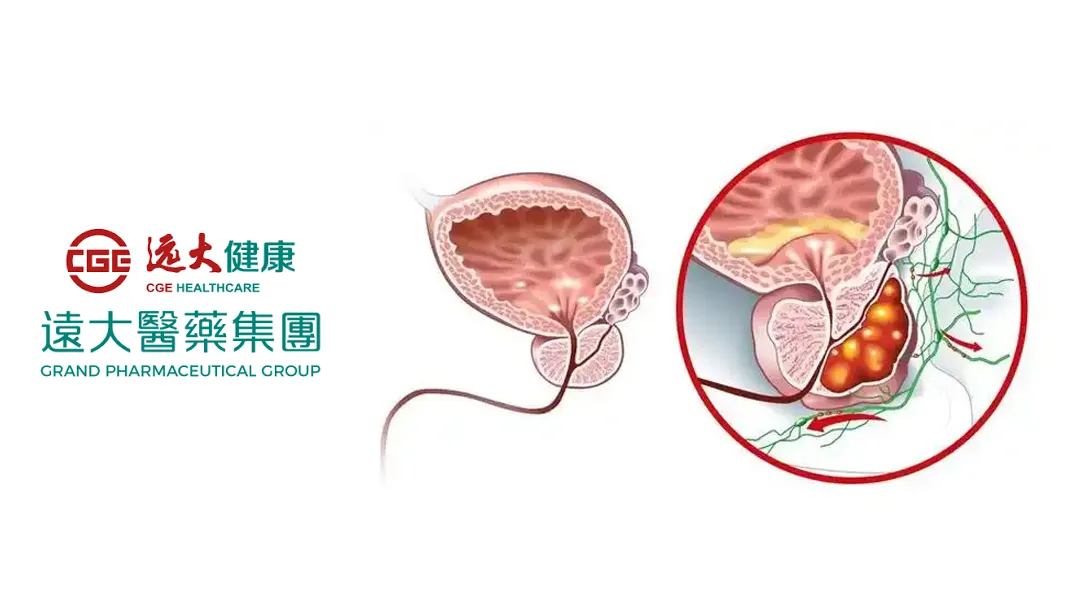
China-based Grand Pharmaceutical Group Limited (HKG: 0512) announced that a filing to conduct a Phase III clinical study for its radionuclide drug conjugate (RDC) TLX591 (177Lu-HuJ591) has been accepted for review by the National Medical Products Administration (NMPA). The study aims to evaluate the efficacy and safety of TLX591 in combination with standard therapy compared to standard therapy alone in metastatic castration-resistant prostate cancer (mCRPC) patients previously treated with androgen receptor pathway inhibitors (ARPI).
Study Design
The Phase III clinical study is designed as a prospective, randomized, controlled, open-label, global multi-center trial, expected to enroll over 500 patients across China, the US, Australia, and New Zealand. The trial will assess the benefits of TLX591 in combination with standard therapy compared to standard therapy alone.
TLX591 Overview
TLX591 is an RDC carrying therapeutic radioactive isotopes, developed to treat prostate-specific membrane antigen (PSMA) positive mCRPC patients whose disease has progressed after ARPI treatment. The drug requires a dual-dose regimen with an interval of approximately 14 days, significantly shortening the treatment period and improving patient compliance.
Diagnostic Product and Collaboration
TLX591-CDx, a related RDC product for diagnosing prostate cancer, initiated its Phase III clinical study in China in August 2023. Both TLX591 and TLX591-CDx are co-developed by Grand Pharma and its Australian partner Telix Pharmaceuticals Limited (ASX: TLX).-Fineline Info & Tech