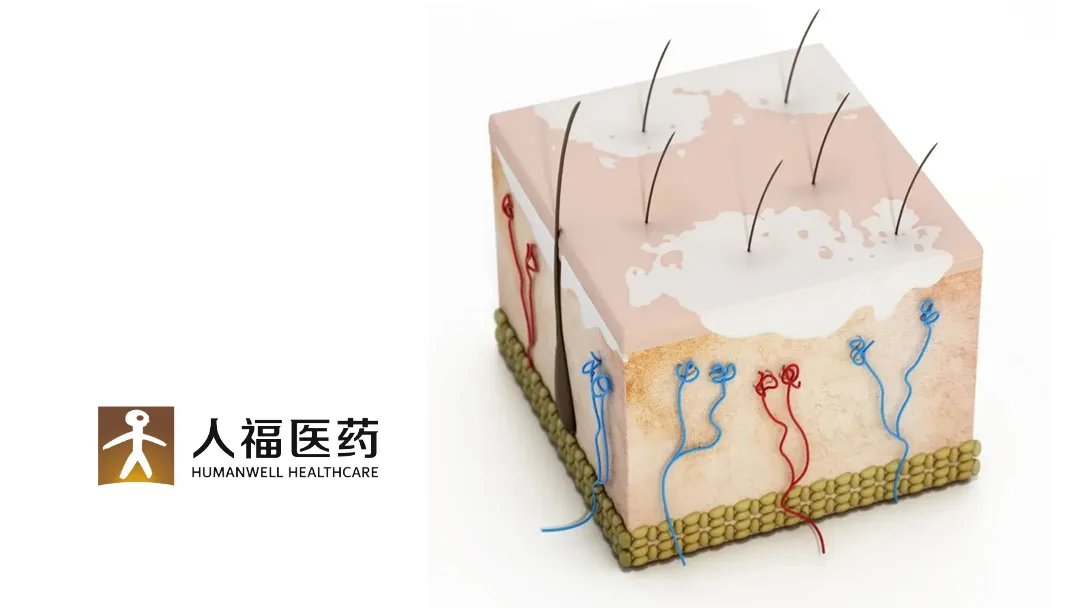
China-based Humanwell Healthcare (Group) Co., Ltd (SHA: 600079) announced that it has received clearance from the National Medical Products Administration (NMPA) to conduct a multi-center, randomized, double-blinded, placebo-controlled Phase II study of its HZ-J001 cream. The study will evaluate the efficacy and safety of the cream in patients with non-segmental vitiligo.
Previous Approvals and Market Landscape
HZ-J001 cream was previously approved for clinical studies in atopic dermatitis, alopecia areata, and vitiligo in China in October 2023. In the domestic market, there are two similar drugs for vitiligo at the Phase II stage, one at the Phase III stage, and another that has been filed for marketing.-Fineline Info & Tech