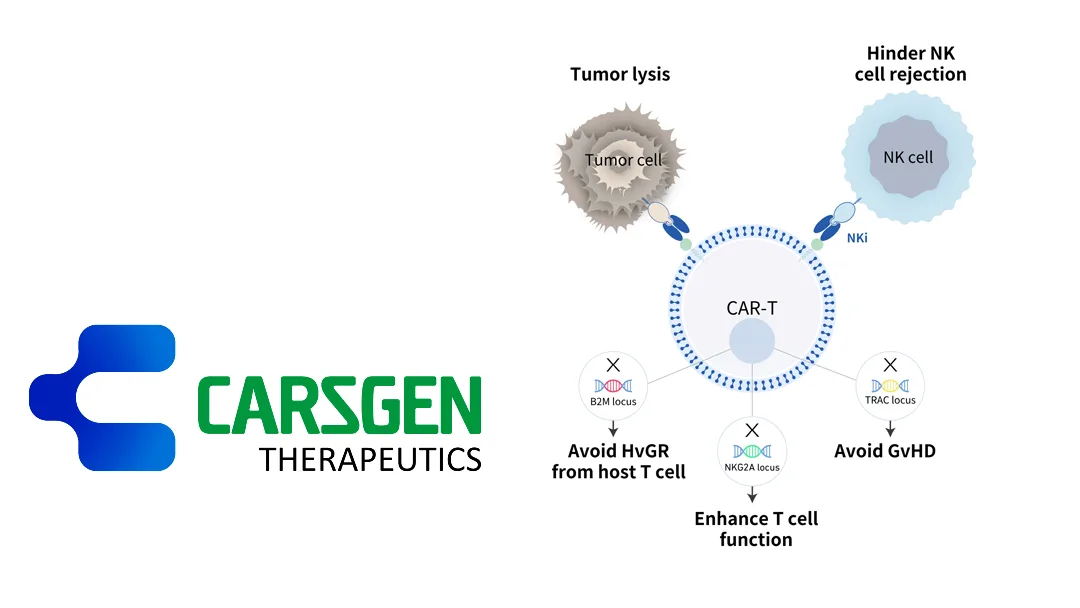
China-based CARsgen Therapeutics Holdings Ltd (HKG: 2171) announced preliminary clinical data for CT0596, an allogeneic BCMA-targeted CAR-T therapy developed using the THANK-u Plus platform. The early exploratory clinical study is evaluating the safety and preliminary efficacy of CT0596 in patients with relapsed/refractory multiple myeloma (R/R MM) or relapsed/refractory plasma cell leukemia (R/R PCL).
Clinical Trial Results
As of May 6, 2025, eight patients with R/R MM who had received at least three prior lines of therapy were enrolled and treated with CT0596 following lymphodepletion with the FC regimen (fludarabine 22.5-30 mg/m² and cyclophosphamide 350-500 mg/m²). During the four-month follow-up period, no dose-limiting toxicities (DLTs), ≥Grade 3 cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), or graft-versus-host disease (GVHD) were reported.
Preliminary Efficacy
Among the five patients who completed the first efficacy assessment at Week 4, three patients (60%) achieved stringent complete response/complete response (sCR/CR), and four patients (80%) achieved minimal residual disease (MRD)-negativity in the bone marrow. All sCR/CR patients continued to maintain their responses, including the first patient who completed the four-month follow-up.-Fineline Info & Tech