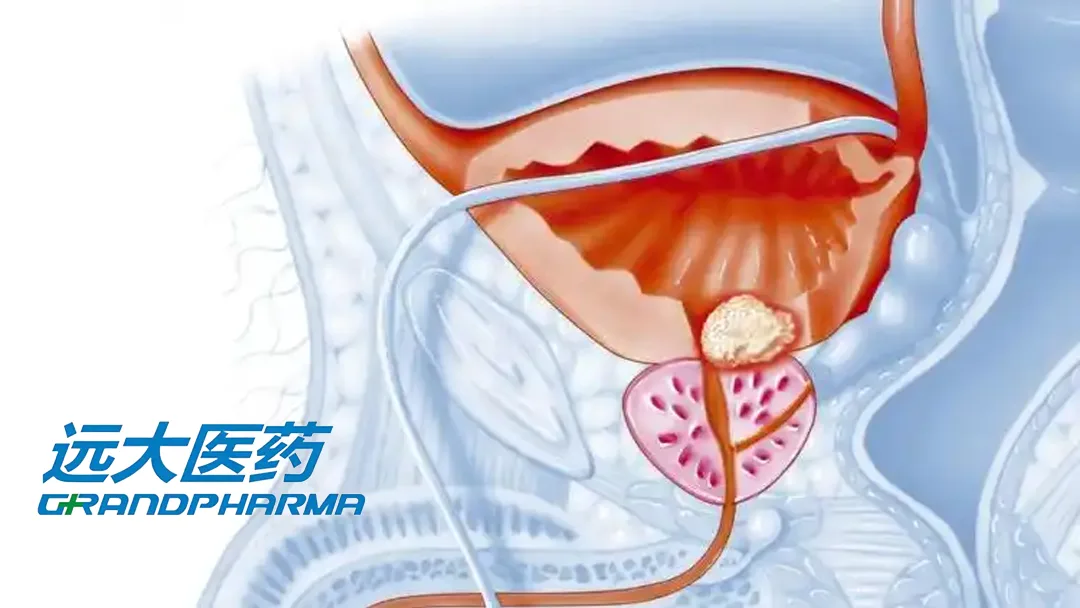
Grand Pharmaceutical Group Limited (HKG: 0512) announced the completion of patient enrollment for the Phase III clinical study of TLX591-CDx (Illucix) in China. This milestone paves the way for the submission of a New Drug Application (NDA) to Chinese authorities within the year.
About TLX591-CDx
TLX591-CDx is a kit for the preparation of Gallium 68 (68Ga) PSMA-11, a radioactive diagnostic agent used for prostate cancer imaging via positron emission tomography (PET). The product is co-developed by Grand Pharma and its Australian partner Telix Pharmaceuticals Ltd (ASX: TLX).
Global Approval Status
The TLX591-CDx has already received approval in Australia, the United States, Canada, multiple European countries, Brazil, and other regions. Its companion product, TLX591, is a radionuclide drug conjugate (RDC) targeting metastatic castration-resistant prostate cancer (mCRPC).-Fineline Info & Tech