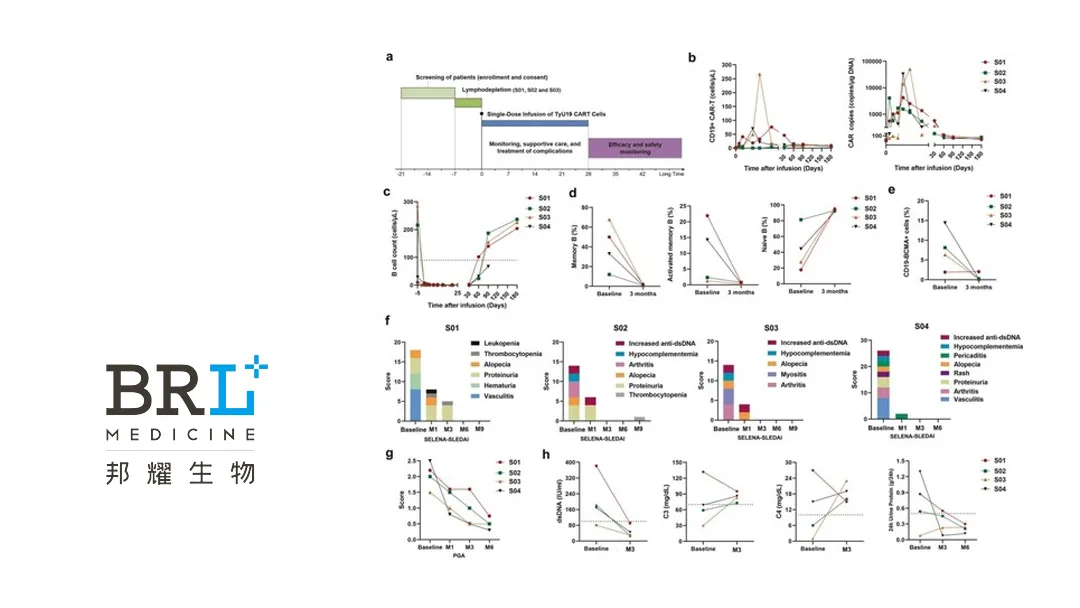
Shanghai-based BRL Medicine Inc. announced positive results from an investigator-initiated trial (ITT) evaluating its allogeneic anti-CD19 CAR-T cells (TyU19) for the treatment of relapsed/refractory systemic lupus erythematosus (SLE). Conducted between September 2023 and September 2024, the trial enrolled four young women aged 22–24 years with refractory SLE. Baseline SELENA-SLEDAI scores ranged from 14 to 26, and all patients had a history of multi-organ involvement, including lupus cerebritis. Notably, none of the patients exhibited active central nervous system lupus at enrollment. Prior therapies included various immunosuppressants and biologics.
Clinical Outcomes
All four patients demonstrated sustained improvement in clinical signs and symptoms. At the 3-month follow-up, all patients met the Systemic Lupus International Collaborating Clinics (SLICC) criteria for a sustained response (SRI-4). All patients achieved a SELENA-SLEDAI score of zero and a PGA score of <1 within 3–6 months. Symptoms such as arthritis, alopecia, and finger vasculitis/ulceration resolved in all patients.
Safety Profile
TyU19 exhibited a favorable safety profile. Only grade 1 cytokine release syndrome (CRS), manifesting as transient fever, was observed in the patients. No cases of immune effector cell associated neurotoxicity syndrome (ICANS) or graft-versus-host disease (GVHD) were reported during the treatment period.-Fineline Info & Tech