China-based WuHan Hamilton Biotechnology Co., Ltd. has announced receiving clinical trial approval from the National Medical Products Administration (NMPA) for its Category 1 biologic product: human umbilical cord mesenchymal stem cells (HUC-MSCs) for the treatment of premature ovarian failure. This marks a significant milestone as the product becomes the first of its kind cleared for study in this indication in China.
Premature Ovarian Failure and Its Challenges
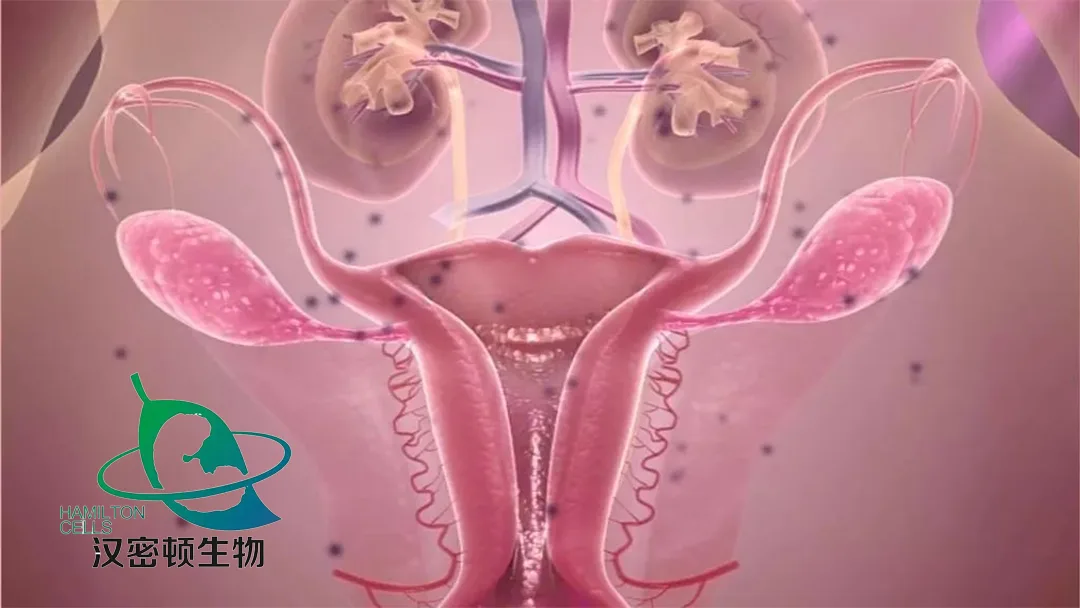
Premature ovarian failure is a condition characterised by menstrual abnormalities, elevated levels of gonadotropins, fluctuating levels of estrogen, and is often accompanied by cardiovascular and neurodegenerative diseases. This approval opens the door to potential new therapies for patients suffering from this complex condition.
Hamilton Bio’s HUC-MSCs Product Mechanism
Hamilton Bio’s HUC-MSCs product offers a novel approach to treating premature ovarian failure. It works by partially restoring ovarian structure and function, thereby improving patients’ quality of life. The product achieves this by inhibiting apoptosis, promoting the proliferation of ovarian granulosa cells, and suppressing local inflammatory reactions within ovarian tissue.-Fineline Info & Tech