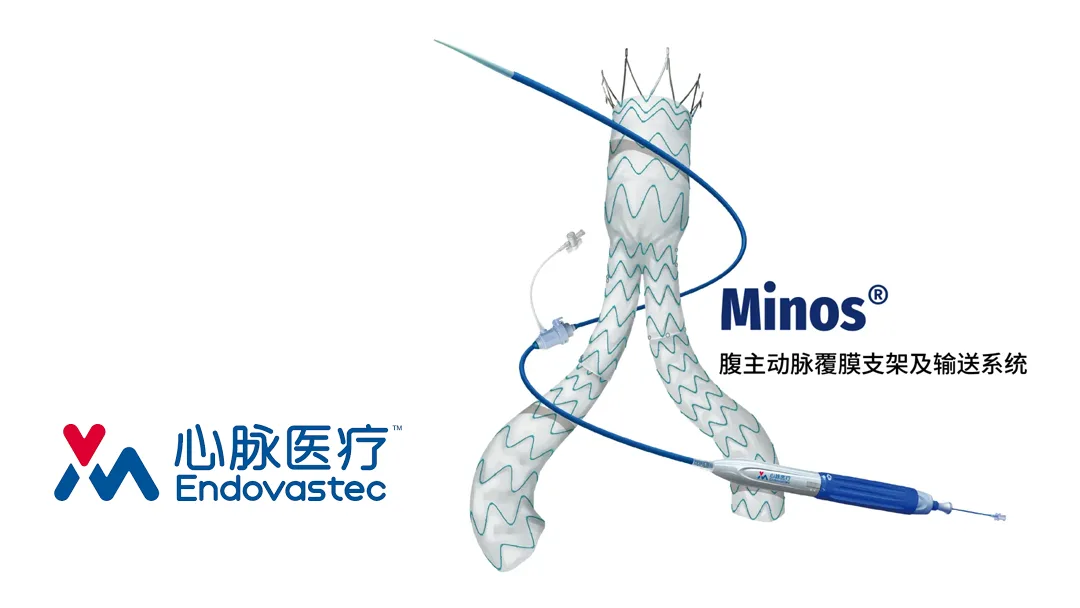
China’s Shanghai MicroPort Endovascular MedTech Co., Ltd (SHA: 688016) has announced that it has received marketing approval in Egypt for its Minos abdominal aortic stent graft and delivery system. This marks the first African market authorization for the company’s internally developed vascular devices.
Product Details and Innovation
The Minos abdominal aortic stent is designed for the endovascular treatment of abdominal aortic aneurysms with a proximal neck length of ≥ 15mm. It is the first domestically developed product to reduce the outer sheath diameter of the delivery device to 14F, significantly lowering the surgical requirements for vascular access. The stent’s bracket structure features a three-piece set design, allowing for flexible assembly within the body and maximizing coverage of the lesion area.
Global Application and Reach
First approved in China in 2019, the Minos stent graft has been applied in clinical settings across 25 countries worldwide. This recent approval in Egypt highlights Shanghai MicroPort’s expanding global presence and commitment to advancing vascular device innovation.-Fineline Info & Tech