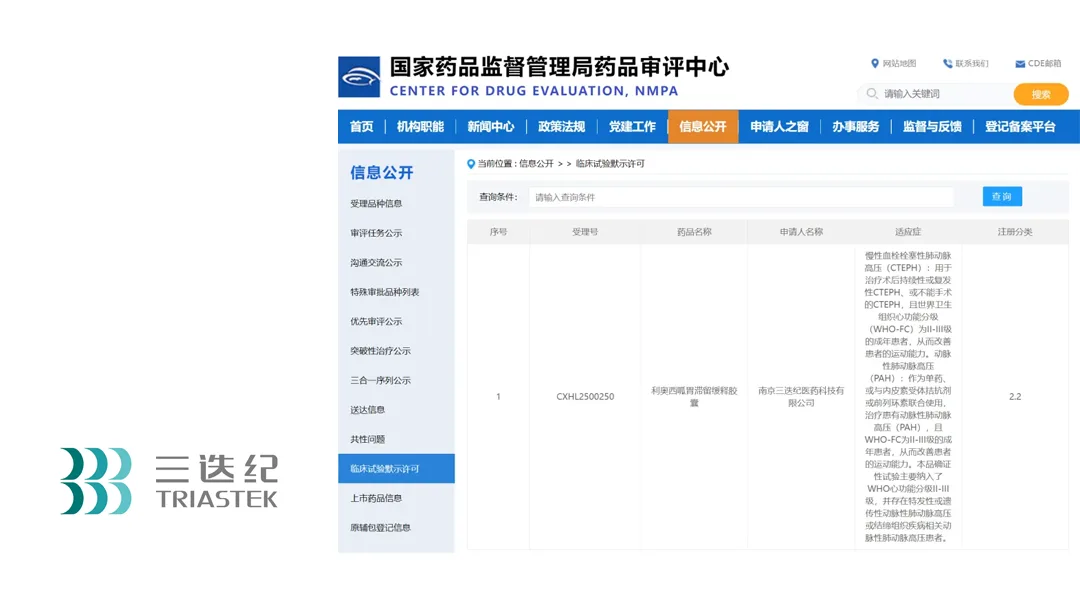
Nanjing-based Triastek Inc., a pioneer in 3D-printed pharmaceutical formulations, has obtained Investigational New Drug (IND) clearance from China’s National Medical Products Administration (NMPA) for its T22 gastric retention sustained-release capsules containing riociguat. This follows the product’s earlier IND authorization from the U.S. Food and Drug Administration (FDA) in January 2023.
Riociguat and T22 Development
Riociguat is currently the only targeted therapy approved for the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension (PAH). T22, a Category 2.2 modified new drug developed using Triastek’s innovative MED&MIM technology, features a petal-shaped design that enables stable gastric retention. This novel formulation reduces riociguat dosing frequency from three times daily to once daily, significantly improving patient compliance.-Fineline Info & Tech