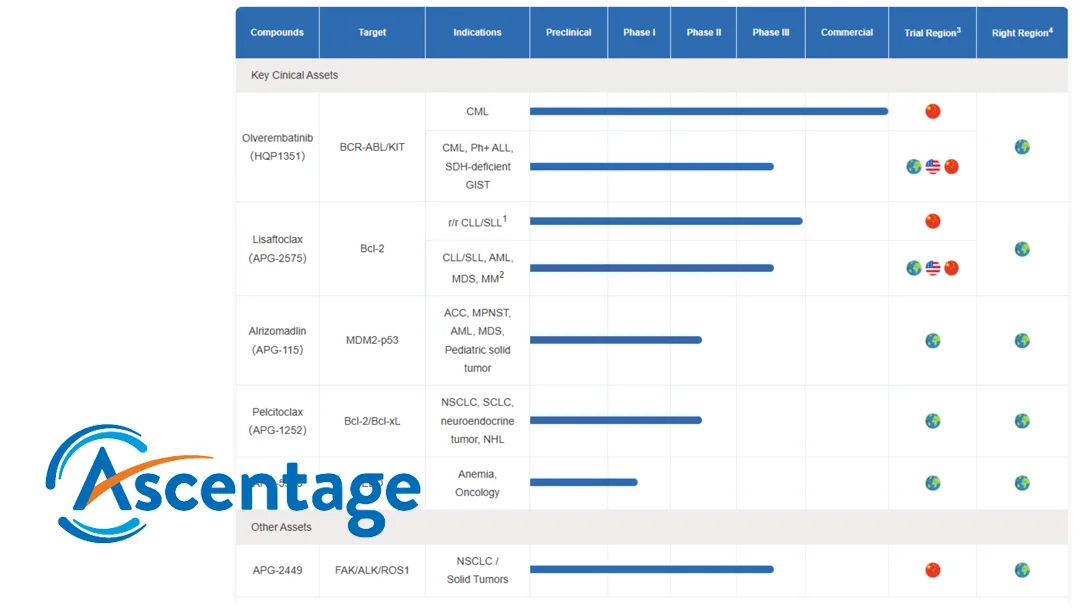
China-based Ascentage Pharma (NASDAQ: AAPG, HKG: 6855) will present the latest data from two ongoing investigational studies evaluating lisaftoclax in various blood cancers and alrizomadlin in solid tumors at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting. These key drug candidates are part of Ascentage’s apoptosis-targeted pipeline.
Lisaftoclax Study Updates
Lisaftoclax, a novel Bcl-2 inhibitor for the treatment of various hematological malignancies, will have its Phase Ib/II study results updated. The study evaluated the drug in patients with treatment-naïve (TN) or prior venetoclax-exposed myeloid malignancies when combined with azacitidine (AZA). Results showed an overall response rate (ORR) of 64% in patients with TN-myelodysplastic syndromes (MDS)/chronic myelomonocytic leukemia (CMML), with complete response (CR) and marrow CR achieved by 29% and 36% of patients, respectively. In patients with diseases refractory to venetoclax, the ORR was 17% in patients with acute myeloid leukemia (AML)/mixed phenotype acute leukemia (MPAL) and 50% in patients with high-risk MDS.
Alrizomadlin Study Results
APG-115, a MDM2-p53 inhibitor, will present results from a Phase II study with or without toripalimab in patients with advanced adenoid cystic carcinoma (ACC) or other solid tumors. Results showed an ORR of 22.2% and a disease control rate (DCR) of 100% in 9 patients with ACC. The DCR was 100% in all patients with malignant peripheral nerve sheath tumor (MPNST), all 5 of whom achieved stable disease (SD). The ORR was 16.7% and DCR was 66.7% in 6 patients with liposarcoma (LPS). The ORR was 14.3% and DCR was 53.6% in patients with MPNST. Overall adverse reactions were controllable, regardless of whether APG-115 was used as monotherapy or in combination therapy.-Fineline Info & Tech