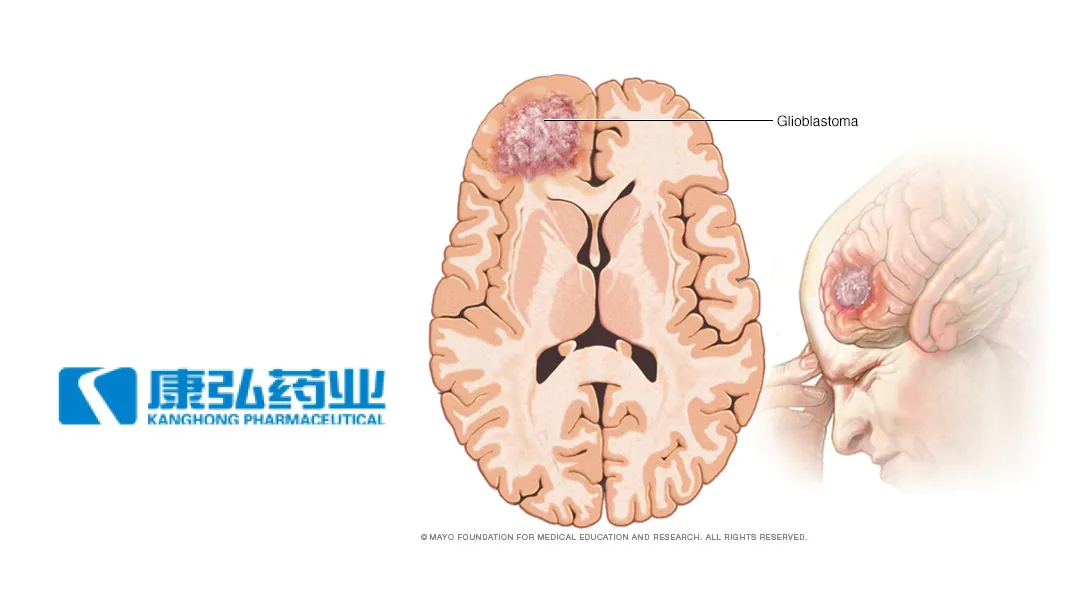
China-based Chengdu Kanghong Pharmaceutical Group Co., Ltd (SHE: 002773) announced that it has received clinical trial approval from the National Medical Products Administration (NMPA) for its Category 1 chemical drug KH617. The approval allows the drug to be used in combination with standard therapy for newly diagnosed glioblastoma.
KH617 and Its Development
KH617 is the first drug developed through Kanghong Pharma’s synthetic biology platform to enter clinical trials. It has demonstrated good safety and tolerability, along with preliminary anti-tumor efficacy signals, in Phase I trials.-Fineline Info & Tech