China-based Beijing Mabworks Biotech Co., Ltd announced that its New Drug Application (NDA) for MIL62, a third-generation anti-CD20 antibody, has been accepted for review by the National Medical Products Administration (NMPA). The application targets the treatment of neuromyelitis optica spectrum disorders (NMOSD), a rare autoimmune disorder of the central nervous system.
NMOSD Background
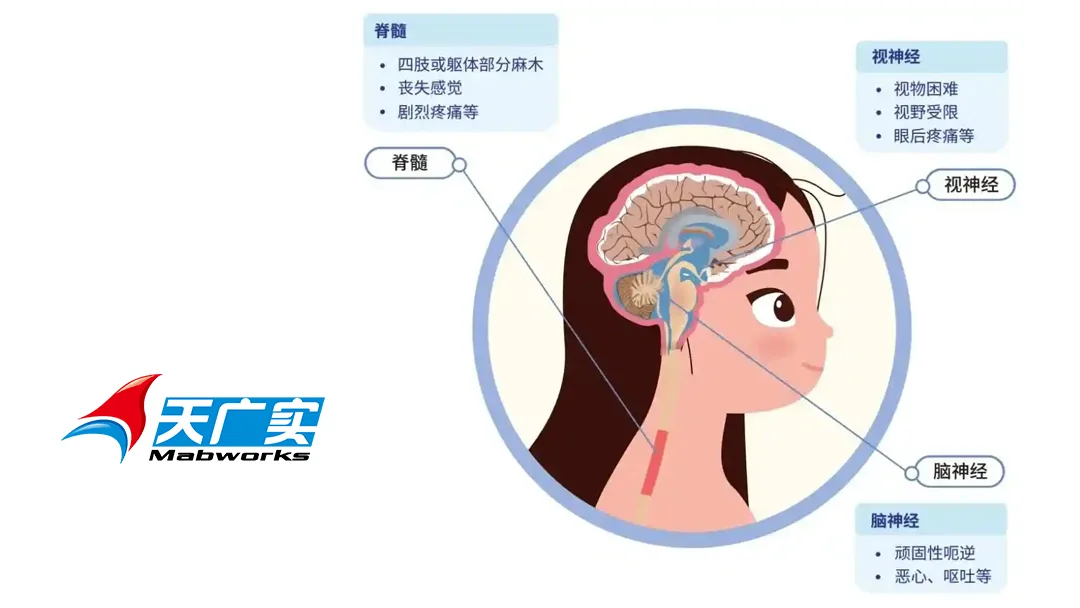
NMOSD predominantly affects young adults, particularly women, and is characterized by high relapse and disability rates. Severe cases can lead to vision loss and paralysis. In 2018, NMOSD was included in China’s first list of rare diseases by the National Health Commission. Despite the availability of three imported biologics for NMOSD treatment in China, their high cost limits patient accessibility, and no domestically developed drugs have been approved yet.
MIL62 as a Potential Solution
MIL62 is poised to address this unmet need as potentially the first China-made therapy for NMOSD. Its development aims to provide a more accessible treatment option for patients suffering from this debilitating condition.-Fineline Info & Tech