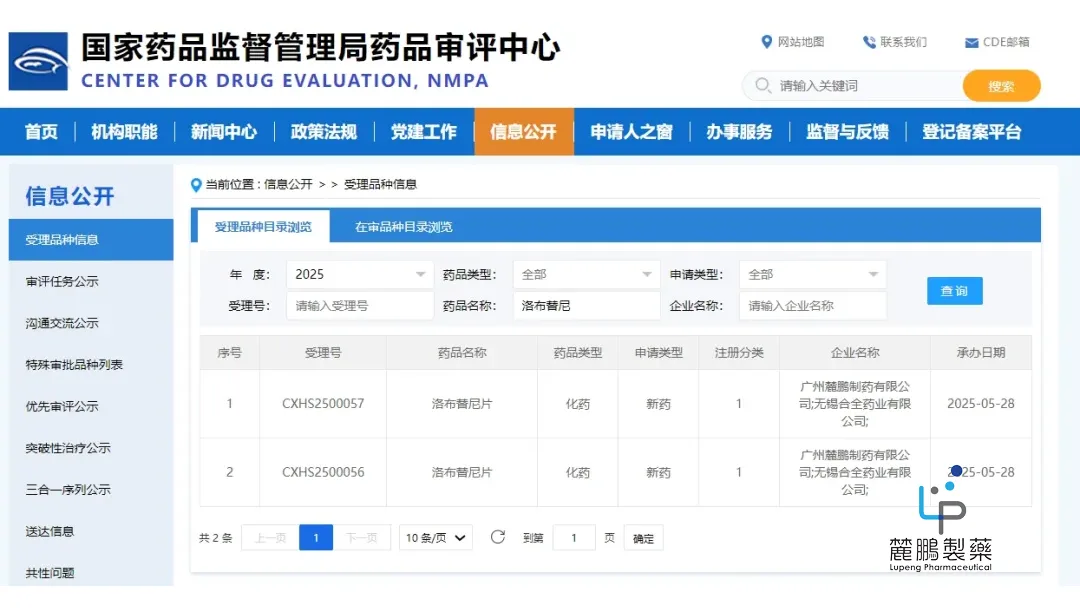
Guangzhou Lupeng Pharmaceutical Co., Ltd’s rocbrutinib is currently under New Drug Application (NDA) review at the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) and has been granted priority review status. The company is seeking approval for its use in adult mantle cell lymphoma (MCL) patients who have previously been treated with Bruton’s tyrosine kinase (BTK) inhibitors.
Rocbrutinib Innovation
Rocbrutinib stands out as the world’s first fourth-generation dual covalent/non-covalent BTK inhibitor. It effectively targets multiple BTK mutations that confer resistance to first, second, and third-generation inhibitors. The drug has demonstrated significant efficacy in clinical studies, particularly in relapsed/refractory non-germinal center B-cell-like diffuse large B-cell lymphoma (R/R non-GCB DLBCL).
Regulatory Milestones and Clinical Trial Progress
Rocbrutinib was awarded Breakthrough Therapy Designation (BTD) in China in May 2024, marking it as the first drug of its kind to achieve this status for DLBCL in the country and globally for BTK inhibitors. Recently, the drug also received clearance for a pivotal Phase II regulatory study targeting R/R non-GCB DLBCL in China.-Fineline Info & Tech