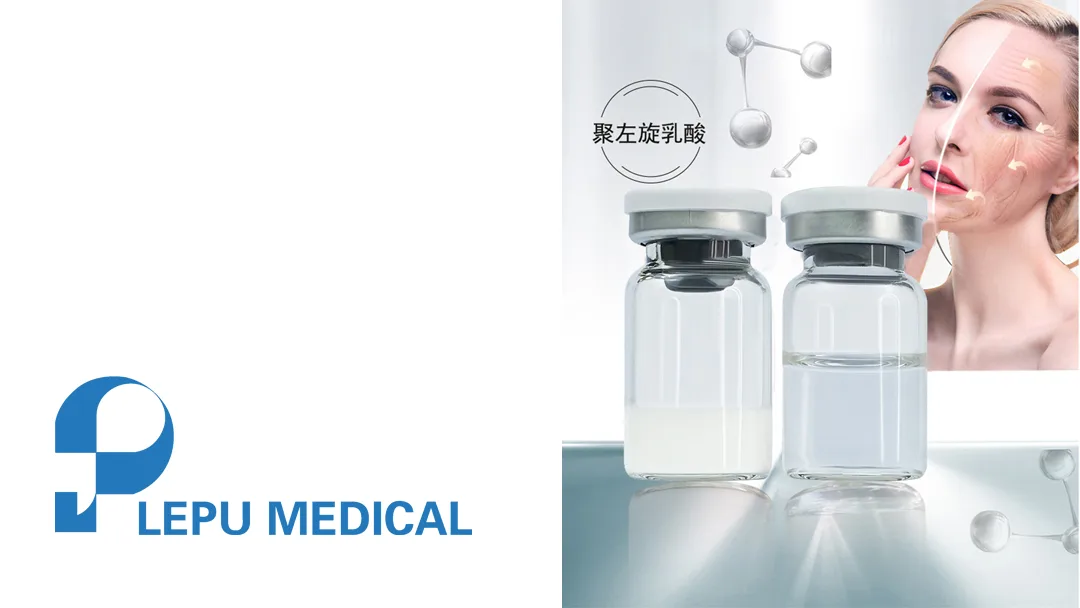
Beijing-based Lepu Medical Technology (SHE: 300003), a provider of cardiovascular disease solutions, announced that its in-house developed polylactic acid facial filler has received Class III medical device registration approval from China’s National Medical Products Administration (NMPA).
Product Details
The polylactic acid facial filler, commonly referred to as a “collagen-stimulating dermal filler,” is an injectable soft tissue augmentation product. It contains poly-L-lactic acid (PLLA), a biocompatible and biodegradable material. When injected into the deep dermis, PLLA stimulates collagen production, effectively correcting facial contours, smoothing wrinkles, and restoring volume in areas with folds or depressions.
Safety and Metabolism
PLLA gradually degrades into carbon dioxide and water, ensuring complete metabolic clearance without leaving long-term residue. This makes the product a safe and effective option for facial contouring and wrinkle reduction.-Fineline Info & Tech