China-based Akeso Biopharma (HKG: 9926) announced that it has received another indication approval from the National Medical Products Administration (NMPA) for its bispecific antibody (BsAb) drug candidate cadonilimab (AK104). This approval allows cadonilimab to be used in combination with platinum with or without bevacizumab for first-line persistent, recurrent, or metastatic cervical cancer. This addresses an unmet need for immunotherapy in first-line cervical cancer patients in China and marks the third broad-population indication approved for cadonilimab.
Drug Mechanism and Approval
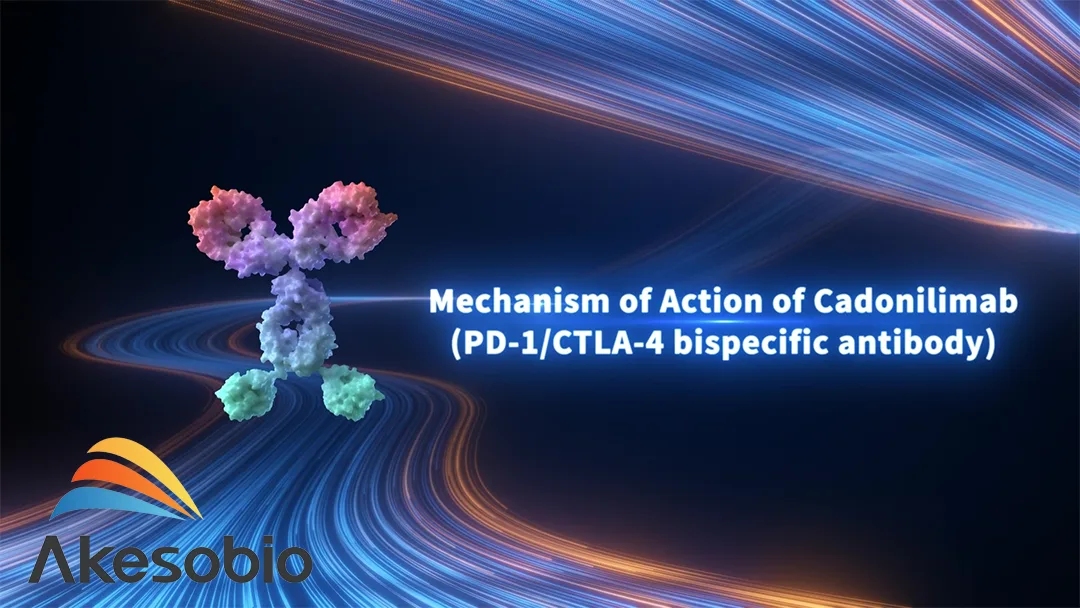
Cadonilimab targets both programmed death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). It was first conditionally approved in China in June 2022 for patients with recurrent or metastatic cervical cancer who had previously failed platinum-based chemotherapy.
Previous Approvals
In September of last year, cadonilimab received marketing clearance for use in combination with chemotherapy for first-line unresectable, locally advanced, recurrent, or metastatic gastric or gastroesophageal junction (GC/GEJ) adenocarcinoma in China.-Fineline Info & Tech