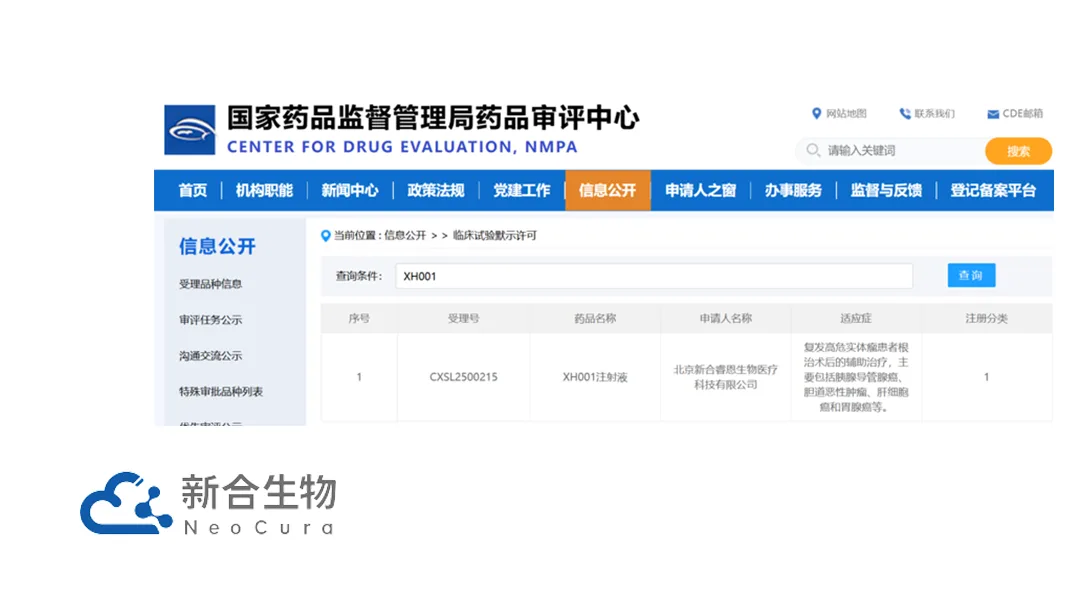
China-based NeoCura Bio-Medical Technology Co., Ltd. announced that it has received approval from the National Medical Products Administration (NMPA) to initiate a clinical study for its XH001, an mRNA personalized neoantigen cancer vaccine. This marks a significant step forward in the development of innovative cancer therapies in China.
AI-Driven Vaccine Development
XH001 is NeoCura Bio’s first AI-driven mRNA personalized neoantigen vaccine. Developed using the company’s proprietary NeoCura AI ALPINE system, it screens for highly immunogenic neoantigens. By integrating patient-specific tumor mutation profiles and HLA genotyping data, the vaccine delivers custom-coded neoantigens designed to activate tumor-specific T cells and precisely eliminate residual cancer cells.
Mechanism and Benefits
This approach aims to reduce the risk of cancer recurrence by targeting residual cancer cells. The vaccine has the potential to significantly impact cancer treatment by providing a personalized and precise method to combat residual cancer cells.-Fineline Info & Tech