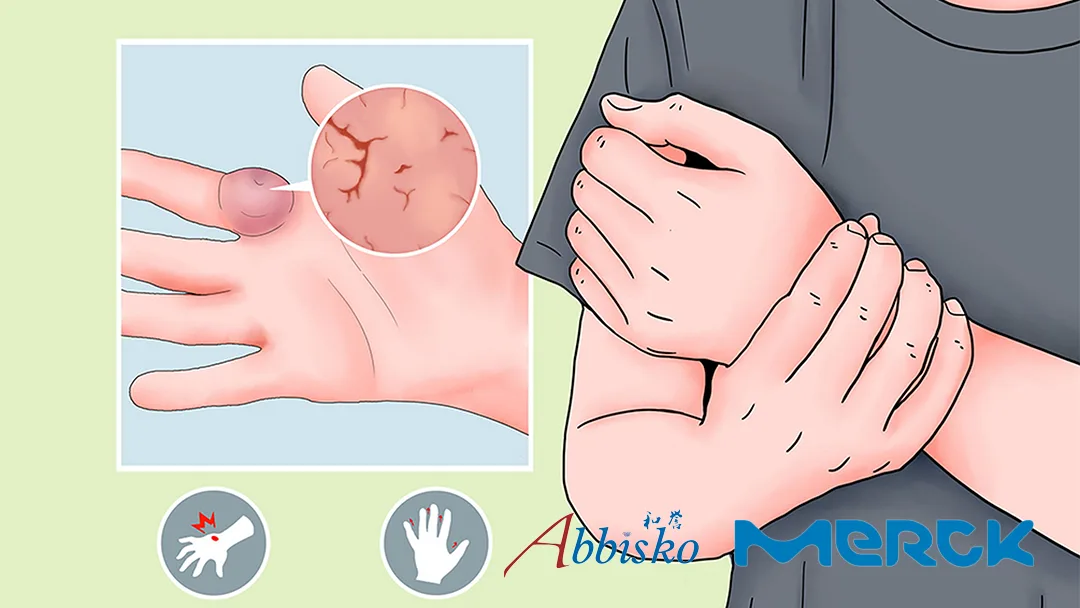
German pharmaceutical major Merck KGaA (ETR: MRK) announced that the New Drug Application (NDA) for pimicotinib has been accepted for review by China’s Center for Drug Evaluation (CDE). The drug is indicated for adult patients with tenosynovial giant cell tumor (TGCT) requiring systemic therapy. Pimicotinib previously received Priority Review and Breakthrough Therapy designations from the CDE.
Clinical Trial Results
The NDA submission is based on the global Phase III MANEUVER study, which achieved its primary endpoint. At week 25, the objective response rate (ORR) in the pimicotinib group was 54.0%, significantly higher than the placebo group’s 3.2%. Pimicotinib demonstrated statistically and clinically significant improvements in key secondary endpoints related to patient-reported outcomes (PRO), including enhanced range of motion, physical function, and reduced stiffness and pain.
Global Licensing Agreement
Pimicotinib is a potentially best-in-class CSF-1R inhibitor developed by Abbisko Therapeutics (HKG: 2256). Merck initially entered a USD 600 million licensing agreement with Abbisko in December 2023 for commercial rights in Mainland China, Hong Kong, Macau, and Taiwan. In April this year, Merck exercised an option to obtain worldwide commercialization rights for pimicotinib.-Fineline Info & Tech