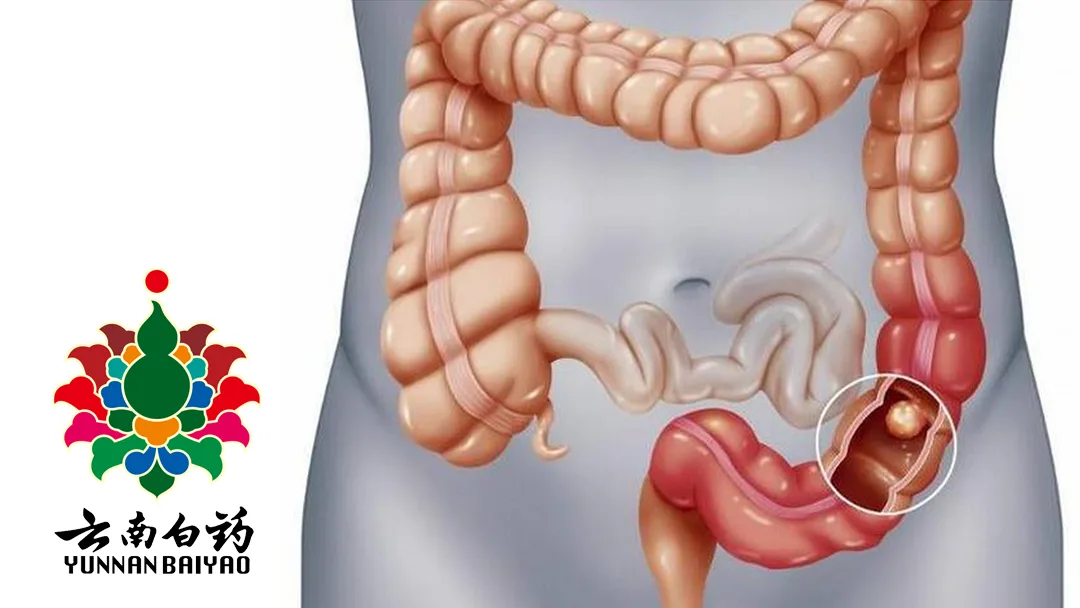
Kunming-based Yunnan Baiyao Group Co., Ltd (SHE: 000538), traditionally known for Chinese medicine, announced clinical trial clearance from the National Medical Products Administration (NMPA) for JZ-14 of its subsidiary Yunbaiyao Zhengwu Technology (Shanghai) Co., Ltd. This Category 1 chemical drug is set to be assessed for treating ulcerative colitis.
Innovative Mechanism
JZ-14 is a potential first-in-class small molecule immunomodulator. It selectively binds to specific protein targets and inhibits downstream signaling pathways, including AKT/NF-κB.
Clinical Promise
The drug has demonstrated significant immunomodulatory and anti-proliferative activity in ulcerative colitis and various tumor models.
Global Patent Protection
The core compound patents for JZ-14 have been granted in China, the US, and Europe. Target-specific and indication patents have completed PCT filings and domestic applications.-Fineline Info & Tech