China-based Shandong Xinhua Pharmaceutical Co., Ltd (SHE: 000756) announced the initiation of a Phase II clinical study for its investigational Class 1 chemical drug OAB-14 dry suspension. The trial targets mild-to-moderate Alzheimer’s disease (AD).
Drug Mechanism and Development
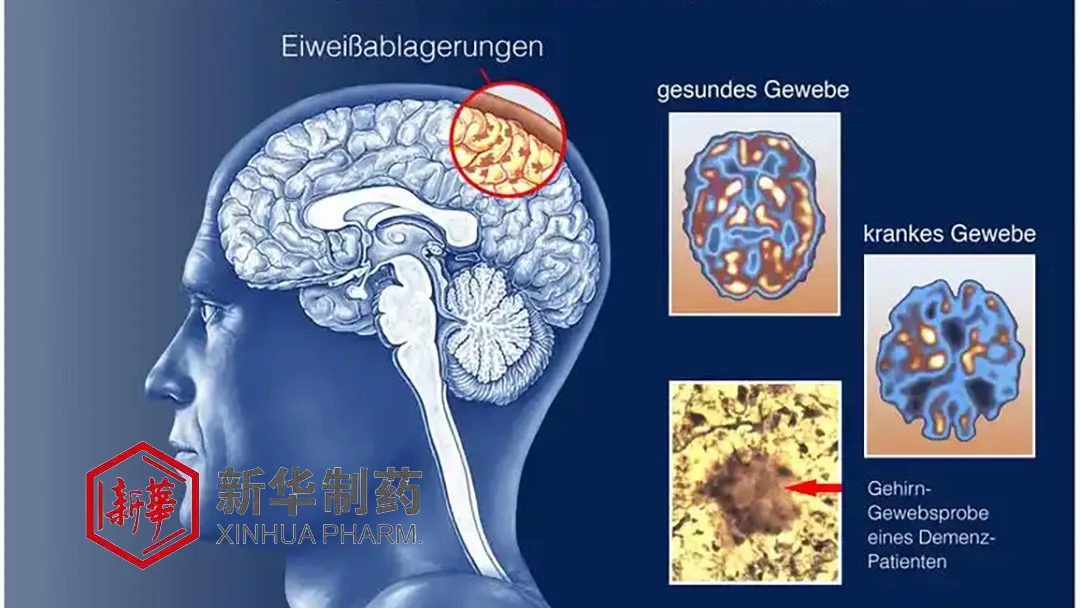
OAB-14, co-developed by Xinhua Pharma and Shenyang Pharmaceutical University, can significantly reduce the deposition of cerebral β-amyloid plaque in the brain. It also decreases the excessive phosphorylation of Tau protein and protects the structure and function of cortical and hippocampal neurons and synapses. These actions aim to improve cognition in AD patients.
Clinical Progress
Phase I results have confirmed OAB-14’s favorable safety and tolerability in healthy adult subjects. The first Chinese patient has been successfully enrolled in the Phase II clinical study.-Fineline Info & Tech