Denmark-based pharmaceutical company Lundbeck A/S (OTCMKTS: HLUBF) announced that it has received Orphan Drug Designations (ODDs) from the US FDA in May and the European Medicine Agency (EMA) in June for its investigational drug Lu AG13909. The designations are for the treatment of congenital adrenal hyperplasia (CAH).
Drug Mechanism
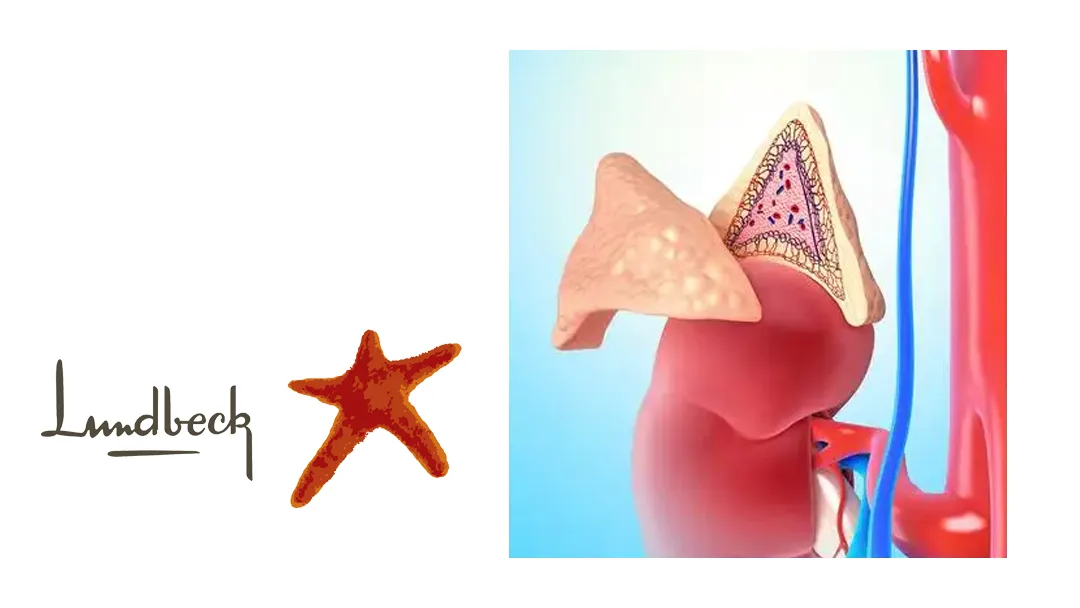
Lu AG13909 is a humanized anti-ACTH monoclonal antibody (mAb) that binds with high affinity to adrenocorticotropic hormone (ACTH). By blocking ACTH’s interaction with the melanocortin 2 receptor (MC2R) in the adrenal glands, it suppresses ACTH-driven neurohormonal signaling, leading to reduced secretion of glucocorticoids, mineralocorticoids, and androgens.
Clinical Trial Progress
The drug is currently being evaluated in an open-label Phase I/II study in adults (18-70 years) with classic CAH. The trial will be conducted across seven countries/regions in North America and Europe, with the first batch of sites scheduled to open by the end of June 2025. Participants will receive intravenous medication every month and undergo an extension period evaluation of up to 12 months.
Safety Profile
Preclinical studies have shown that continuous administration of Lu AG13909 for six months resulted in no toxic reactions.-Fineline Info & Tech