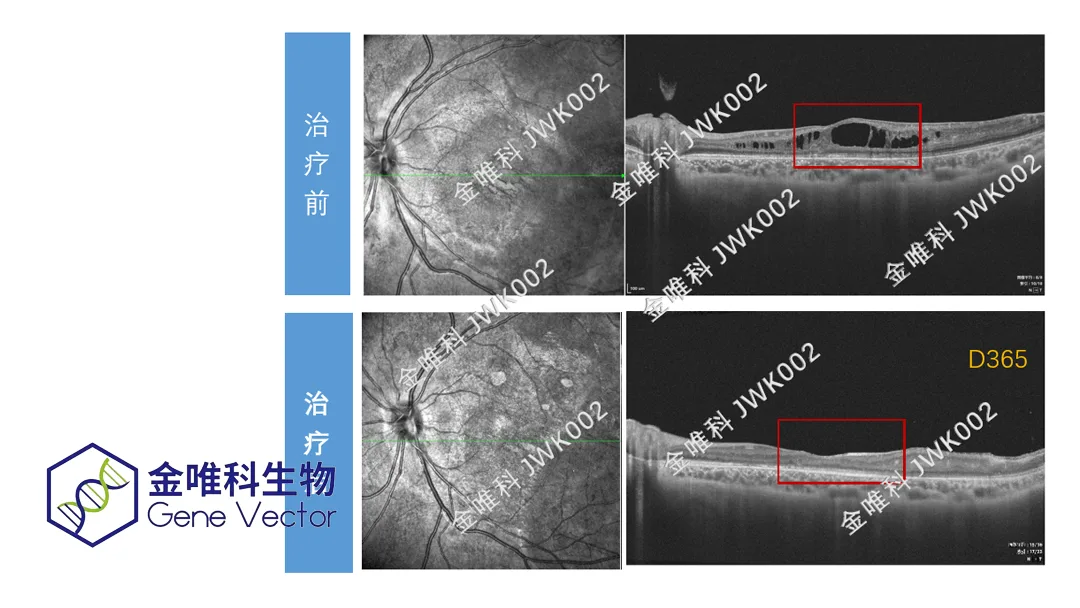
China-based Chengdu Geneector Biotechnology Co., Ltd. (Genevector), a developer of AAV gene therapy drugs, announced that it has received Investigational New Drug (IND) approval from the National Medical Products Administration (NMPA) for its Category 1 gene therapy JWK002 targeting X-linked retinoschisis (XLRS). This marks the first time a gene therapy drug for XLRS has entered regulatory clinical trials in China.
Regulatory and Designation Milestones
JWK002 has previously received Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD) from the U.S. FDA.
Disease Background
X-linked retinoschisis (XLRS) is an X-linked recessive genetic disorder caused by mutations in the retinoschisin 1 (RS1) gene. It is characterized by varying degrees of vision loss, visual field defects, macular schisis, and reduced b-wave amplitude on electroretinography (ERG). Primarily affecting males, XLRS is typically diagnosed in early childhood. The severity of visual impairment varies among patients, and severe cases may lead to complications such as retinal detachment and vitreous hemorrhage. Current clinical management mainly involves regular monitoring, carbonic anhydrase inhibitors, and treatment for complications, with no effective curative therapies available.
Gene Therapy Mechanism
JWK002 is an adeno-associated virus (AAV)-based gene therapy. It enables efficient restoration of RS1 protein expression in retinal cells through systematic optimization of tropic serotype selection and gene expression elements. This approach aims to improve retinal structure and function in patients.-Fineline Info & Tech