China-based Angel Pharmaceuticals Ltd announced that it has received approval from the National Medical Products Administration (NMPA) to initiate a Phase Ib/II clinical study in China. The trial will assess the safety and efficacy of its soquelitinib (CPI-818) in patients with atopic dermatitis (AD). The study will also determine two optimal dosing regimens.
Drug Profile
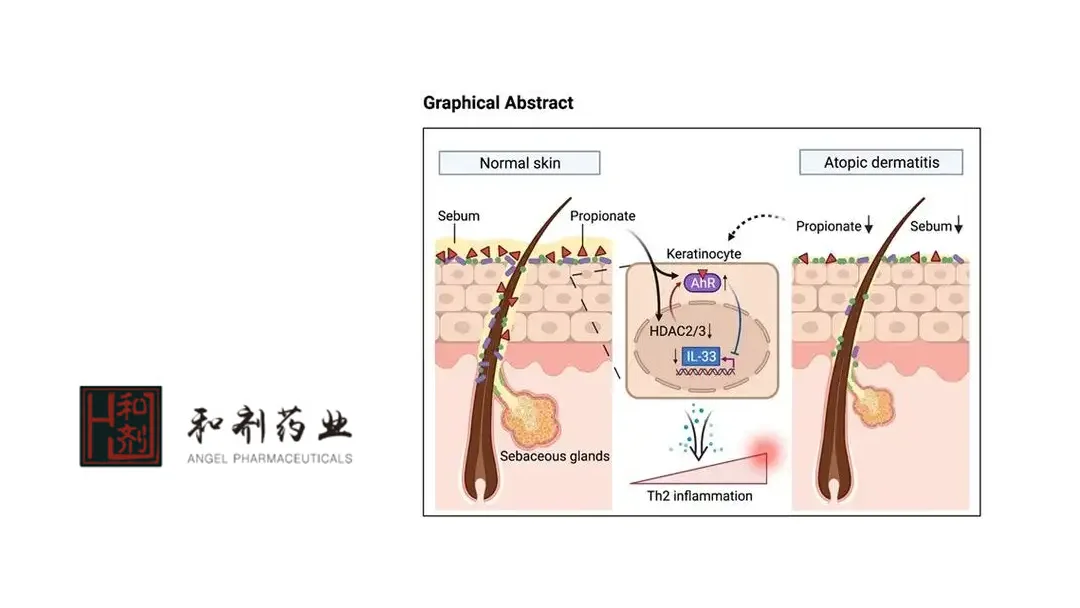
Soquelitinib is a highly selective oral small-molecule interleukin-2-inducible T-cell kinase (ITK) inhibitor. Unlike current injectable biologic therapies that target only single or limited cytokines, soquelitinib can block multiple cytokines involved in inflammatory processes. This mechanism has the potential to significantly improve the treatment landscape for atopic dermatitis.-Fineline Info & Tech