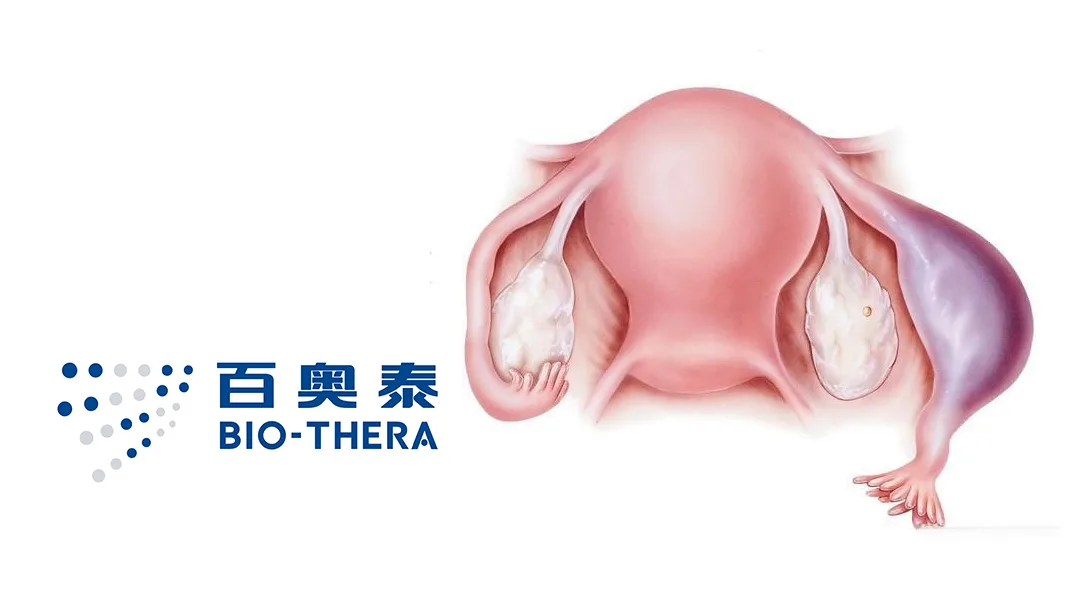
Guangzhou-based Bio-Thera Solutions (SHA: 688177) announced the successful first patient dosing in its Phase III clinical study of BAT8006, a folate receptor α (FRα)-targeting antibody-drug conjugate (ADC) for platinum-resistant ovarian cancer. This milestone marks significant progress in addressing a challenging clinical condition with limited treatment options.
Clinical Challenge
Platinum-resistant ovarian cancer is associated with a poor prognosis and limited therapeutic choices. Currently, only one FRα-targeting ADC, mirvetuximab soravtansine, has been approved globally. Its use is restricted to patients with FRα expression ≥75%, representing just 25%-30% of the platinum-resistant population. This therapy has shown limited median progression-free survival (mPFS) and is linked to ocular toxicity.
BAT8006 Innovation
BAT8006, one of the first FRα-targeting ADCs in China to enter pivotal Phase III trials, has the potential to deliver clinically meaningful efficacy across all platinum-resistant ovarian cancer patients, regardless of FRα expression levels. Promising data suggest that BAT8006 may offer a novel treatment option for this difficult-to-treat patient group.-Fineline Info & Tech