China-based Jiangsu Hengrui Pharmaceuticals Co., Ltd. (SHA: 600276) announced that it has received marketing approval from the National Medical Products Administration (NMPA) for its Aihengping, a generic version of Pacira’s Exparel (bupivacaine liposome). The drug is now approved for regional postoperative analgesia via popliteal sciatic nerve block and adductor canal block in adults.
Drug Profile
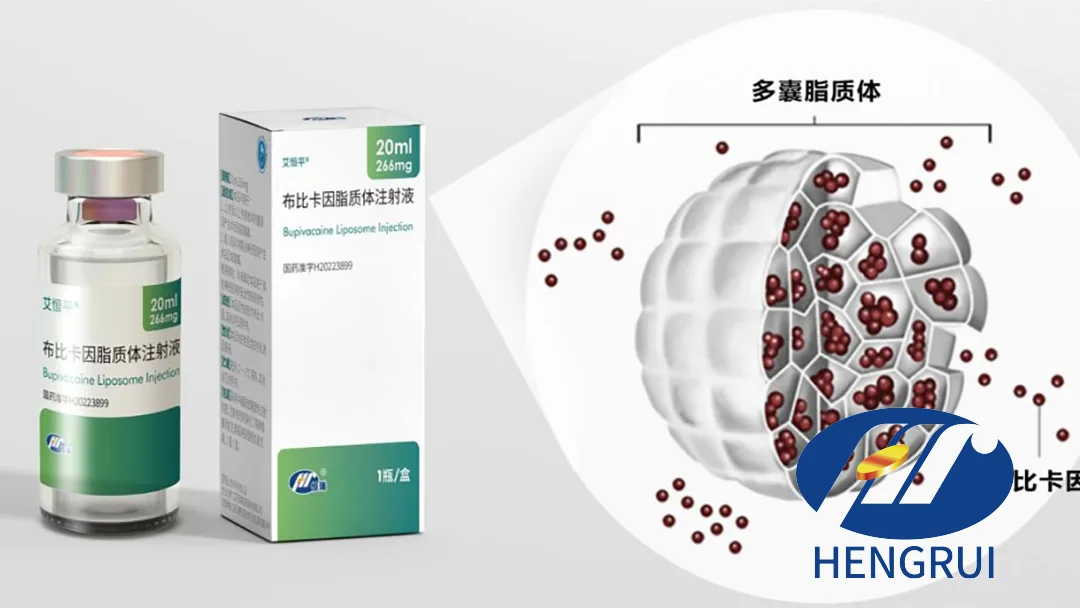
Aihengping is an ultra-long-acting local anesthetic that extends the duration of analgesia from the conventional 5-6 hours of bupivacaine to several days. This is achieved through an advanced multivesicular liposome drug delivery system, offering significant improvements in pain management for surgical patients.
Previous Approvals and Global Expansion
The drug was previously approved for single-dose infiltration induced postoperative local analgesia in patients aged 12 and above, as well as for postoperative regional analgesia in adult patients undergoing intermuscular groove brachial plexus block. Aihengping, as the first generic version of Exparel, received Chinese approval in late 2022 and obtained FDA clearance in July 2024, marking a significant step in its global market expansion.-Fineline Info & Tech