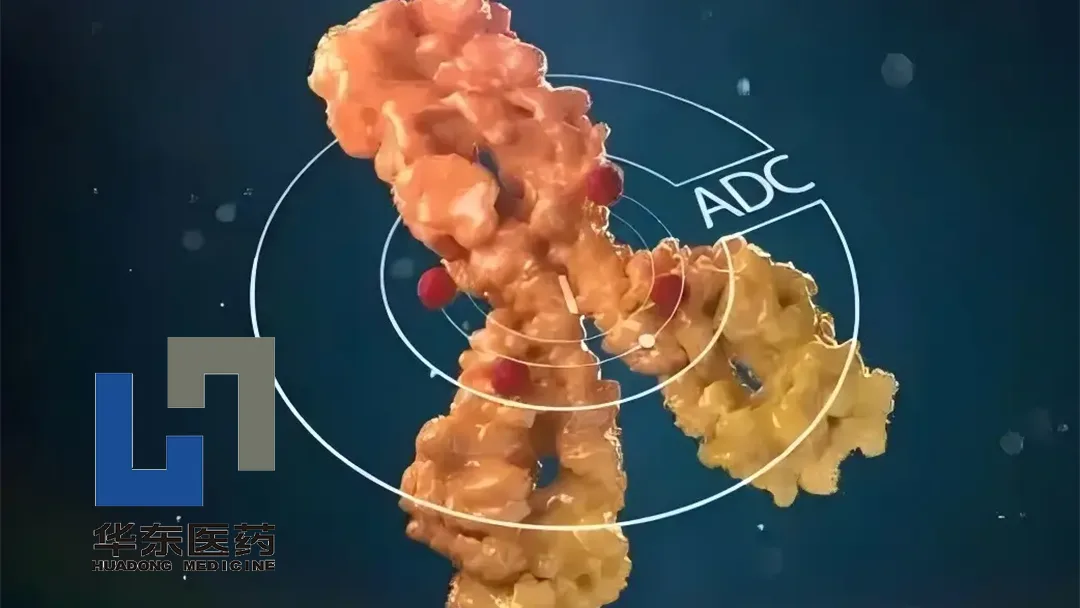
China-based Huadong Medicine Co., Ltd (SHE: 000963) announced that it has received clearance from the US Food and Drug Administration (FDA) to conduct a Phase I study for its first-in-class antibody-drug conjugate (ADC) HDM2012. This novel therapy is designed for the treatment of advanced solid tumors.
Drug Profile
HDM2012 is a first-in-class ADC targeting MUC-17 (Mucin-17). The drug comprises an anti-MUC-17 monoclonal antibody, a topoisomerase I inhibitor payload, and a cleavable linker with a drug-to-antibody ratio (DAR) of 8. This design aims to deliver targeted therapy with enhanced precision and efficacy.
Preclinical Results
Preclinical studies demonstrated that HDM2012 possesses favorable drug properties, safety, and efficacy. It exhibited potent antitumor activity across various tumor models and showed good tolerability in animal experiments.-Fineline Info & Tech